L. Li1, J. Baek1, B.M. Jesdale1, A.L. Hume2, G. Gambassi3, R.J. Goldberg1, K.L. Lapane1
1. Department of Quantitative Health Sciences, University of Massachusetts Medical School, 368 Plantation Street, Worcester, MA 01605, USA; 2. University of Rhode Island College of Pharmacy, 7 Greenhouse Road, Kingston, RI 02881, USA; 3. Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy and Università Cattolica del Sacro Cuore, Largo Agostino Gemelli 8, 00168 Rome, Italy. Corresponding author: Kate L. Lapane, PhD, University of Massachusetts Medical School, 368 Plantation Street, Albert Sherman Center, 6-1083 Worcester, MA 01605, Email: kate.lapane@umassmed.edu
Jour Nursing Home Res 2019;5:60-67
Published online November 13, 2019, http://dx.doi.org/10.14283/jnhrs.2019.11
Abstract
Background: Despite the growing importance of skilled nursing facility care for Medicare patients hospitalized with heart failure, no risk prediction models for these patients. Objectives: To develop and validate separate predictive models for 30-day all-cause mortality and 30-day exist all-cause re-hospitalization. Design: Retrospective cohort study using nationwide Medicare claims data cross-linked with Minimum Data Set 3.0. Setting: 11,529 skilled nursing facilities in the United States (2011-2013). Participants: 77,670 hospitalized heart failure patients discharged to skilled nursing facilities (randomly split into development (2/3) and validation (1/3) cohorts). Measurements: Using data on patient sociodemographic and clinical characteristics, health service use, functional status, and facility-level factors, we developed separate prediction models for 30-day mortality and 30-day re-hospitalization using logistic regression models in the development cohort. Results: Within 30 days, 6.8% died and 24.2% were re-hospitalized. Thirteen patient-level factors remained in the final model for 30-day mortality and 10 patient-level factors for re-hospitalization with good calibration. The area under receiver operating characteristic curves were 0.71 for 30-day mortality and 0.63 for re-hospitalization in the validation cohort. Conclusions: Among Medicare patients with heart failure discharged to skilled nursing facilities, our predictive model based on administrative data may be used to identify those at risk for dying within 30 days, which could aid clinicians and family members in improving care for patients with heart failure during this vulnerable period. Further work identifying factors for re-hospitalization remains needed.
Key words: Heart failure, skilled nursing facility, risk prediction, mortality, re- hospitalization.
Introduction
Heart failure (HF) currently affects ~6.5 million American adults (1), and is the leading cause of hospitalization among persons aged ≥65 years (2). Nearly one-quarter of Medicare beneficiaries are discharged to skilled nursing facilities (SNFs) after being hospitalized for HF (3, 4). These patients are typically older, and have greater mortality and re-hospitalization risks compared with those discharged to home (4). SNFs play a critical role in the care continuum of patients with HF, from transitions from the hospital to the SNF and then from SNFs back to patients’ residences (5). The SNF care use for patients with HF has steadily increased in recent decades, which may be partially due to changes in Medicare reimbursement policies (6).
Little is known about factors that predict short-term mortality and re-hospitalization in high-risk SNF patients. Studies have examined the risks of dying or being re-hospitalized among patients with HF (7-11); no models exist specifically for patients with HF discharged to SNFs (12). Older patients with HF are particularly vulnerable during the 30-day period after being discharged from the hospital after an episode of HF (13). Understanding predictors of 30-day outcomes at the time of admission to a SNF could guide the intensity of clinical monitoring, frequency of patient reassessments, and the extent of clinical intervention needed (12). We developed and internally validated predictive models for 30-day mortality and re-hospitalization risks.
Methods
Medicare Provider Analysis and Review (MedPAR) files were linked to Minimum Data Set (MDS) 3.0, Provider of Services files, Nursing Home Compare Data, and the Medicare Beneficiary Summary Files (MBSF) for 2011-2013. MedPAR includes administrative and clinical elements obtained from discharge abstracts for hospitalizations and SNF stays of fee-for-service beneficiaries. The MDS is a federally- mandated comprehensive clinical assessment of residents living in all Medicare/Medicaid certified nursing facilities. It captures resident-level items including demographic characteristics, functional status, and diagnoses. For SNF residents, the standard Centers for Medicare and Medicaid Services (CMS) assessment occurs on days 5, 14, 30, 60, and 90 (14). The reliability and validity of MDS 3.0 items including residents’ medical, cognitive, functional, and psychological status has been confirmed (15, 16). The MBSF contains beneficiaries’ demographic, enrollment, and death related information. The University of Massachusetts Medical School Institutional Review Board approved the study.
We identified all episodes of an acute hospitalization with ≥3 consecutive days of stay (17) with a primary diagnosis of HF (18) discharged to a SNF on the day of or day after the index hospital discharge (Appendix Figure 1). For those with multiple eligible episodes, one was randomly selected.
Andersen’s behavioral model of health services (19) guided the selection of potential patient-level predictor variables with a focus on predisposing and need factors. Predisposing factors included patient’s age, gender, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, or non-Hispanic other), marital status (married, widowed, separated/divorced, or never married), and dual eligibility status (Medicare and Medicaid dual enrollment or Medicare only). Need factors were comorbidities, health service use, health characteristics, and functional status. We considered SNF characteristics in our modeling approaches because facility associated factors (e.g., nurse staffing) are associated with outcomes of care (12). Potential predictors with <5% prevalence or ≥10% missingness were excluded.
Comorbidities included 2 acute (pneumonia and urinary tract infection based on the MDS) and 11 chronic diagnoses (coronary artery disease, atrial fibrillation, cerebrovascular disease, peripheral vascular disease, hyperlipidemia, diabetes, chronic obstructive pulmonary disease (COPD) or asthma, anemia, chronic kidney disease (stage ≥3), dementia, and depression in 6 months before index date). We calculated a Charlson index (20). We classified patients as having systolic, diastolic, and unspecified HF based on the primary diagnosis at the time of the index hospitalization (18).
Health service use need factors were based on acute hospitalization claims: length of the index hospital stay, stay in an intensive care unit during the patient’s index hospitalization (yes/no), number of hospitalizations in 6 months before the index hospitalization (0, 1, ≥2), number of hospitalizations for HF (0, 1, ≥2), and for other heart diseases (0, 1, ≥2).
Health characteristic need factors included: body mass index (BMI, <18.5, 18.5-< 25, 25-<30, ≥30 kg/m2), dyspnea, urinary incontinence (continent, incontinent, or not rated [catheter, ostomy or no urine output]), fall history in the month before the index SNF admission (yes, no, or unable to determine), pressure ulcers (stage ≥1), presence of pain (yes/no), and level of depression severity based on the Patient Health Questionnaire (PHQ)-9 or PHQ-9 Observational Version. Cut-points of 5, 10, 15, and 20 defined none, mild, moderate, moderately severe, and severe depression, respectively (21).
Functional status need factors included an assessment of patient’s physical limitations and cognitive impairment. Physical limitations was assessed based on the MDS activities of daily living score (22) and categorized as either normal or minimal limitations (0-2), moderate limitations (3-4), or physical dependency (5-6). We included variables for six individual activities such as eating, dressing, and bathing. Each activity was rated on a scale from independence, supervised, limited assistance, extensive assistance, or total dependency (including only did activity once or twice/didn’t do activity in the past 7 days). Cognitive impairment was measured based on the self- reported Brief Interview for Mental Status or a staff-reported Cognitive Performance Scale (normal or minimal impairment, moderate impairment, severe impairment) (23-25).
We included 5-star overall quality rating, 5-star nursing staffing rating, certified bed size (<100, 100-299, ≥300 beds), chain membership (Yes/No), profit orientation (for profit/non-profit), geographic location (urban or rural county), hours per resident per day of total nursing care (quartiles), and hours per resident per day of licensed nursing care (quartiles).
We determined 30-day all-cause mortality based on MBSF. Using MedPAR data, 30-day re-hospitalization was defined as any new non-elective acute care hospitalization, excluding admissions for rehabilitation, within 30 days after the index SNF admission.
We randomly split the sample into a development cohort (two-thirds) and a validation cohort (one-third). The validation cohort was used to test the discrimination ability of the prediction models.
We examined continuous factors including patient age, Charlson index, and length of index hospital stay for possible nonlinear effects through a generalized additive model (GAM) in relation to 30-day mortality and re-hospitalizations separately. Based on the GAM results, we modeled age and log-transformed Charlson index as a linear function for mortality and as a non-parametric function using a smoothing spline for the re-hospitalization outcome. The length of the index hospital stay was categorized as 3-4, 5-6, 7-9, 10-13, ≥14 days based on the prediction curves for both outcomes. To estimate β-coefficients for age and Charlson index for the 30-day re-hospitalization outcome, we also modeled age as 8 categories, i.e., <65, 65-69, 70-74, 75-79, 80-84, 85-89, 90-94, ≥95 years, and Charlson index as ≤4, 5-7, >7 categories.
We set two criteria for variable selection; i) p-value <0.1 and ii) area under the receiver operating characteristic curve (AUC, i.e., C-statistic) increase >0.01 (26). To assess predictive factors for the study outcomes, we sequentially investigated and added covariates to the logistic regression models (Models 1-6), beginning with predisposing factors (Model 1), next adding comorbidity need factor variables (Model 2), need factor variables for health service use (Model 3), need factor variables for health characteristics (Model 4), need factor variables for functional status (Model 5), and then facility-level variables (Model 6). We examined the clustering effects of SNFs as random effects of SNFs in the final model for each outcome and evaluated whether AUCs were improved.
Calibration was assessed using the Hosmer and Lemeshow (H-L) test for all models without the random effects of SNFs (p >0.05 indicating good calibration).
Because using the H-L test in a hierarchical logistic regression model may not be valid due to correlation within clusters, we visually assessed the extent of agreement between observed and predicted values. Predicted values of each outcome were sorted in ascending order and equally divided into 20 groups. We considered an indication of lack-of-fit when the differences between observed and predicted values were not random.
For validity, we calculated the AUCs for each model in the validation cohort. An AUC of 0.5 indicates no discrimination and 1 represents perfect discrimination. We consider AUCs of 0.60-0.69 to indicate limited discrimination, AUCs of 0.70 – 0.79 to indicate modest discrimination, and AUCs ≥0.80 to indicate good discrimination (27). In addition, to compare the contribution to prediction power for each factor in the final model, we calculated the percentage of decrease in prediction power if the corresponding factor was absent in the development cohort using the following formula: (AUC (Final model)-AUC (Model without corresponding factor))/(AUC (Final model)-0.5)*100%. The percent of decrease is 100% when a model only includes the intercept.
Sensitivity analyses were conducted to examine the robustness of the predictive models. We estimated the AUCs in models with all predictors which were associated with outcomes and had positive contributions to the AUCs. We estimated AUCs in the completed cases without missing data. We estimated the AUCs in patients aged ≥65 years, stratified by sex. We estimated AUCs for 30-day re-hospitalization among patients who survived 30 days after SNF admission. R 3.4.0 with mgcv package for GAM models and gamm4 package for a generalized additive mixed model (www.r-project.org) were used.
Results
From 11,529 SNFs, 51,783 were in the development and 25,887 in the validation cohort (see Appendix Figure 1 for patient selection details). In the development cohort, the median age of patients was 84 years, 39.6% were men, 83.4% were non-Hispanic whites, and 19.1% were dually enrolled in Medicaid. The median Charlson index was 4 and the median length of index hospital stay was 6 days. Forty-three percent had dyspnea and 24.6% had depression. Eighty percent had moderate physical limitations or dependency and 35.9% had moderate or severe cognitive impairment. Approximately one-half lived in a SNF with a 4- or 5- star rating for registered nurse staffing. Both patient-level and facility-level characteristics of the validation cohort were similar to those in the development cohort (Tables 1 – 2, Appendix Table 1).
After the SNF admission, 6.8% of patients died and 24.2% were re-hospitalized within 30 days in the development cohort. Of those who died, 56.1% were re- hospitalized and 13.6% were discharged to home or self-care before their death. Of those who were re-hospitalized, 18.5% were discharged to home or self-care before their readmission. In the validation cohort, 6.9% died and 24.3% were re-hospitalized within 30 days after the SNF admission similar to those in the development cohort.

Table 1
Predisposing factors, comorbidities, and health service use of the development and validation cohorts
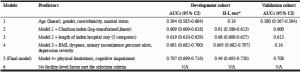
The AUC for 30-day mortality in the development cohort in Model 1 with all predisposing factors except Medicare and Medicaid dual eligibility was 0.594. Adding the log-transformed Charlson index increased the AUC to 0.609; no other comorbidities were selected in Model 2. Adding the length of index hospital stay increased the AUC to 0.619; no other health service use factors were selected in Model 3. Adding BMI, dyspnea, urinary incontinence, having a pressure ulcer, and depression severity further increased the AUC to 0.691 in Model 4. Adding physical limitations and cognitive impairment increased the AUC to 0.707 in Model 5. None of the facility-level factors was selected in Model 6. There was no evidence of a lack of fit in all models based on the H- L tests. The AUCs for each model in the validation cohort were similar to those in the development cohort (Table 3).
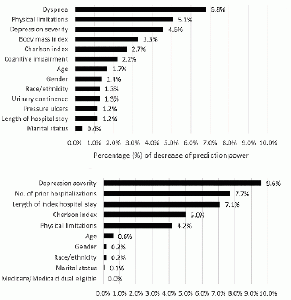
Although adding the random effects of SNFs in Model 5 increased the AUC to 0.750 in the development cohort, the AUC was only 0.709 in the validation cohort. The observed and predicted values were visually close to each other in Model 5, regardless of taking into account the random effects of SNFs or not in the validation cohort (Appendix Figure 2). Because adding the random effects of SNFs in Model 5 did not markedly improve the AUC and calibration in the validation cohort, we selected Model 5 without random effects of SNFs as the final model for 30-day mortality; the β- coefficients for each predictor are listed in Appendix Table 2. Among the selected predictors, developing symptoms of dyspnea, having physical limitations, depression severity, BMI (decreased mortality risk in patients with BMI ≥25) and Charlson index contributed most to prediction power (Figure 1).
The AUC for 30-day re-hospitalization in the development cohort in Model 1 was 0.553. Adding the log-transformed Charlson index increased the AUC to 0.583; no other comorbidities were selected in Model 2. Adding the length of index hospital stay and the number of hospitalizations during the past 6 months increased the AUC to 0.607 in Model 3. Depression severity in Model 4 further increased the AUC to 0.621. Adding physical limitations slightly increased the AUC to 0.626 in Model 5. None of the facility-level factors was selected in Model 6. The p values for the H-L tests were >0.05 for all models. The AUCs for each model in the validation cohort were similar to those in the development cohort.
Adding the random effects of SNFs in Model 5 increased the AUC to 0.667 in the development but not in the validation cohort (AUC = 0.625). The visual agreement between observed and predicted values in Model 5 with random effects of SNFs was not as good as that in Model 5 without the inclusion of random effects of SNFs (Appendix Figure 2). Model 5 without inclusion of the random effects of SNFs was selected as the final model for 30-day re-hospitalization; the β-coefficients for each predictor except age and Charlson Index are listed in Appendix Table 3A. When age and Charlson index were remodeled as categorical variables, the AUC (0.623) was almost the same as the original one and the β-coefficients for each predictor are listed in Appendix Table 3B. Among the selected predictors, depression severity, number of hospitalizations in past 6 months, length of the index hospitalization, Charlson index, and physical limitations contributed most to prediction power (Figure 1).
Top: 30-day mortality model; Bottom: 30-day re-hospitalization model
All sensitivity analyses yielded similar AUCs for 30-day mortality and for 30-day re-hospitalization as those in the original analyses (see Appendix Table 4).
*H-L test: Hosmer and Lemeshow test (p >0.05 indicating good calibration)
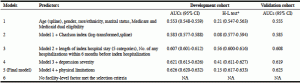
Table 4
Area under receiver operating characteristic curves (AUCs) for 30-day all-cause re-hospitalization
*H-L test: Hosmer and Lemeshow test (p >0.05 indicating good calibration)
Discussion
Routinely collected administrative patient-level data can be used to predict 30-day mortality with modest predictive accuracy and 30-day re-hospitalization with more limited predictive accuracy. Some patient-level factors including dyspnea, physical limitations, depression severity, number of prior hospitalizations, and length of index hospital stay were important predictors for either 30-day mortality or 30-day re- hospitalization, or both. No facility-level factors predicted these outcomes.
Most of HF prediction models/risk scores, including CMS’s (28), predict 30-day mortality after hospital admission (thus including deaths during hospitalization) (9). A limited number of recent studies that have attempted to predict 30-day post-discharge mortality in patients with HF demonstrated moderate to good discrimination (C-statistics: 0.66-0.86), but none used national data (29-32). Studies with good discrimination (C- statistics >0.80) included multiple key laboratory and vital sign variables (29, 32) which were not in our data.
Charlson index, length of index hospital stay, depression, and cognitive function are important predictors for 30-day post-discharge mortality in patients with HF (29, 30, 32), and BMI (protective effect of increased BMI) consistently predicts mortality (9). Our study affirmed these findings in a SNF population. Although some comorbidities, such as diabetes, have been included in various prediction models for mortality in patients with HF (10), none of the specific clinical diagnoses contributed to meaningful additional prediction ability when the model included the Charlson index. We found that dyspnea and physical limitations were important predictors for 30-day mortality. Activities of daily living have been shown to be an important prognostic risk factor in elderly patients with HF (33). Both dyspnea and physical limitations are likely proxies for the severity of HF, advanced age, or both.
Models used to predict readmission after HF hospitalization generally demonstrate poorer discrimination than those developed to predict mortality (7, 9); our study is no exception. The CMS risk adjustment model based on Medicare claims data for 30-day readmission in patients with HF had a C-statistic of 0.60 (34); adding clinical data did not improve model discrimination (35). Other models have been developed using administrative data, electronic health records, or patient-reported information to predict 30-day readmission in patients with HF. Most, including several widely-accepted HF mortality risk scores, demonstrated poor or limited discrimination in predicting hospital readmission (C-statistics: 0.54-0.65) (29-31, 36, 37), Huynh et al. developed a model with a C-statistic of 0.80 for 30-day readmission (32), but the C-statistic dropped to 0.73 when validated on a large patient cohort (38). Mahajan et al. recently developed a model with a C-statistic of 0.84 for readmission in patients with HF, but this study was carried out at a single Veterans health care system (n=1,210 men) and included vital signs and laboratory measures in the model (39).
Re-hospitalization among patients discharged from the hospital after HF has been suggested to be the product of a much broader set of forces involving clinical and non-clinical factors (e.g., socioeconomic status, behavioral, and mental health) (29, 30, 32) although results from the Tele-HF study suggested that non-clinical factors are not dominant factors in predicting readmission risk for patients with HF (37). In our study, depression severity contributed most to the prediction ability for 30-day re-hospitalization, followed by the number of hospitalizations in the past 6 months and length of the patient’s index hospital stay. Although physical limitations were a predictor for re-hospitalization in our study, cognitive function was not. In a small study of 156 older patients from 58 SNFs with an acute HF exacerbation, the authors found that re-hospitalized patients were less likely to have dementia (40).
Current health and reimbursement policies have drawn attention to 30-day mortality and re-hospitalization for patients hospitalized with HF in the United States (41). This is a clinically meaningful period that can be strongly influenced by hospital care and early transition to the outpatient setting. Of more than 1 million hospital discharges for HF each year in the United States (1), approximately one-quarter are discharged into SNFs (3, 4). SNFs have emerged as an integral component of care for Medicare beneficiaries with HF (12). We previously reported that SNF residents with HF are old and suffer from significant physical limitations and cognitive impairment and a high degree of comorbidity (18). Therefore, predictive models of 30-day mortality and re-hospitalization in this highly vulnerable population could potentially serve as a powerful tool for clinicians and patients to make informed decisions and appropriate care planning and coordination.
Despite this, all-cause re-hospitalization is far more difficult to predict and to prevent than cause-specific re-hospitalizations, given the diversity of possible hospitalization diagnoses (42). The discrimination differences between the mortality and re-hospitalization models in our study reaffirm the known challenges in predicting patient risk of re-hospitalization. Furthermore, the differences in predictors between the 30-day mortality and re-hospitalization models suggest that mortality and re-hospitalization may reflect different domains of care.
To our knowledge, this is the first study to develop and validate risk prediction models for patients with HF discharged to SNFs using national data sources. Both prediction models may provide estimates of risk that can assist clinicians in counseling patients and their families and guide a potentially shared clinical decision making process. Furthermore, some modifiable risk factors such as depression and dyspnea have been identified as important predictors, which could be potential targets for intervention or management among this vulnerable population during this high-risk period.
Despite these study strengths, there are several limitations that must be kept in mind in interpreting our study results. First, our models lacked key clinical data such as ejection fraction findings and laboratory values that were not included in administrative data. Adding clinical data to administrative data significantly improved the prediction of mortality among patients hospitalized with HF, although it did not improve the re-hospitalization model (35). Second, the MDS 3.0 did not include information on the use of do not resuscitate orders. Third, the re-hospitalization model did not take into account the competing risk due to death. However, the discrimination capacity for re-hospitalization did not change when we restricted our analyses to those who survived 30 days after their index SNF admission. Finally, although we took into account some SNF-level factors, we did not have information available about HF-specific transitional care and HF disease management approaches. That the AUCs markedly increased after taking into account random effects of SNFs in the development cohort suggests this possibility. Because 18% of SNFs contributed a single patient with HF, these facilities in the validation cohort were not presented in the development cohort, which probably explain that the predictive power in the validation cohort was not improved with random effects of SNFs.
Conclusions
Older patients with HF discharged to SNFs are a diverse population (43). Preventing death and hospital readmission among these patients is challenging. Despite the limitations of currently available data, our model for mortality may be used to identify those at risk for dying within 30 days, which could aid clinicians and family members in improving the care of patients with HF during this vulnerable period. Although readmission within 30 days of a prior hospitalization for HF has emerged as a major focus of quality improvement and payment reform, predicting re-hospitalization is a more daunting task than predicting mortality. Since the model for re-hospitalization had limited discrimination, there is a need for further understanding of the factors that influence re-hospitalization risk.
Acknowledgments: None
Funding: Dr. Li conducted this work while a post-doctoral fellow at the University of Massachusetts Medical School. She now works in the pharmaceutical industry. The other authors have no conflicts to report. This work was funded by grants from the National Institutes of Health (T32HL120823, R01HL35434, R01HL135219, R01 HL125089, R01 HL126911, U01 HL105268 and U01 HL138631).
Conflict of interest: None of the authors have conflicts of interest to disclose. Dr. Lin Li conducted this work while a post-doctoral fellow at the University of Massachusetts Medical School before she began working for the pharmaceutical industry.
Ethical standard: This study was approved by the Institutional Review Board of the University of Massachusetts Medical School.
References
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics- 2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146-e603.
2. Hall MJ, DeFrances CJ, Williams SN, Golosinsky A, Schwartzman A. National Hospital Discharge Survey: 2007 summary. Natl Health Stat Report 2010:1-20, 24.
3. Dolansky MA, Xu F, Zullo M, Shishehbor M, Moore SM, RimmAA. Post-acute care services received by older adults following a cardiac event: a population-based analysis. J Cardiovasc Nurs 2010;25:342-349.
4. Allen LA, Hernandez AF, Peterson ED, et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail 2011;4:293-300.
5. Nazir A, Smucker WD. Heart Failure in Post-Acute and Long-Term Care: Evidence and Strategies to Improve Transitions, Clinical Care, and Quality of Life. J Am Med Dir Assoc 2015;16:825-831.
6. Orr NM, Forman DE, De Matteis G, Gambassi G. Heart Failure Among Older Adults in Skilled Nursing Facilities: More of a Dilemma Than Many Now Realize. Curr Geriatr Rep 2015;4:318-326.
7. Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Arch Intern Med 2008;168:1371-1386.
8. Alba AC, Agoritsas T, Jankowski M, et al. Risk prediction models for mortality in ambulatory patients with heart failure: a systematic review. Circ Heart Fail 2013;6:881-889.
9. Rahimi K, Bennett D, Conrad N, et al. Risk prediction in patients with heart failure: a systematic review and analysis. JACC Heart Fail 2014;2:440-446.
10. Ouwerkerk W, Voors AA, Zwinderman AH. Factors influencing the predictive power of models for predicting mortality and/or heart failure hospitalization in patients with heart failure. JACC Heart Fail 2014;2:429-436.
11. Saito M, Negishi K and Marwick TH. Meta-Analysis of Risks for Short-Term Readmission in Patients With Heart Failure. Am J Cardiol 2016;117:626-632.
12. Orr NM, Boxer RS, Dolansky MA, Allen LA, Forman DE. Skilled Nursing Facility Care for Patients With Heart Failure: Can We Make It «Heart Failure Ready?». J Card Fail 2016;22:1004-1014.
13. Krumholz HM. Post-hospital syndrome–an acquired, transient condition of generalized risk. N Engl J Med 2013;368:100-102.
14. Centers for Medicare & Medicaid Services. Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual. 2015.
15. Saliba D, Buchanan J. Making the investment count: revision of the Minimum Data Set for nursing homes, MDS 3.0. J Am Med Dir Assoc 2012;13:602-610.
16. Saliba D, Buchanan J. Development & validation of a revised nursing home assessment tool: MDS 3.0. 2008.
17. CDC Prevention. Medicare coverage of skilled nursing facility care.
18. Li L, Jesdale BM, Gambassi G, Goldberg RJ, Lapane KL. Who are they? Patients with heart failure in American skilled nursing facilities. J Cardiol 2018;71:428-434.
19. Babitsch B, Gohl D, von Lengerke T. Re-revisiting Andersen’s Behavioral Model of Health Services Use: a systematic review of studies from 1998-2011. Psychosoc Med 2012;9:Doc11.
20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-383.
21. Saliba D, DiFilippo S, Edelen MO, Kroenke K, Buchanan J, Streim J. Testing the PHQ-9 interview and observational versions (PHQ-9 OV) for MDS 3.0. J Am Med Dir Assoc 2012;13:618-625.
22. Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci 1999;54:M546-553.
23. Saliba D, Buchanan J, Edelen MO, et al. MDS 3.0: brief interview for mental status. J Am Med Dir Assoc 2012;13:611-617.
24. Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol 1994;49:M174-182.
25. Centers for Medicare & Medicaid Services. Nursing Home Data Compendium 2015 Edition. 2015.
26. Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157-172; discussion 207-212.
27. Ohman EM, Granger CB, Harrington RA, Lee KL. Risk stratification and therapeutic decision making in acute coronary syndromes. JAMA 2000;284:876-878.
28. Krumholz HM, Wang Y, Mattera JA, et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation 2006;113:1693-1701.
29. Amarasingham R, Moore BJ, Tabak YP, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Med Care 2010;48:981-988.
30. Au AG, McAlister FA, Bakal JA, Ezekowitz J, Kaul P, van Walraven C. Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. Am Heart J 2012;164:365-372.
31. Win S, Hussain I, Hebl VB, Dunlay SM, Redfield MM. Inpatient Mortality Risk Scores and Postdischarge Events in Hospitalized Heart Failure Patients: A Community-Based Study. Circ Heart Fail 2017;10.
32. Huynh QL, Negishi K, Blizzard L, Sanderson K, Venn AJ, Marwick TH. Predictive Score for 30-Day Readmission or Death in Heart Failure. JAMA Cardiol 2016;1:362-364.
33. Lo AX, Donnelly JP, McGwin G, Jr., Bittner V, Ahmed A, Brown CJ. Impact of gait speed and instrumental activities of daily living on all-cause mortality in adults >/=65 years with heart failure. Am J Cardiol 2015;115:797-801.
34. Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes 2008;1:29-37.
35. Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circ Cardiovasc Qual Outcomes 2011;4:60-67.
36. Eapen ZJ, Liang L, Fonarow GC, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail 2013;1:245-251.
37. Krumholz HM, Chaudhry SI, Spertus JA, Mattera JA, Hodshon B, Herrin J. Do Non-Clinical Factors Improve Prediction of Readmission Risk?: Results From the Tele-HF Study. JACC Heart Fail 2016;4:12-20.
38. Huynh Q, Negishi K, De Pasquale CG, et al. Validation of Predictive Score of 30- Day Hospital Readmission or Death in Patients With Heart Failure. Am J Cardiol 2018;121:322-329.
39. Mahajan SM, Burman P, Newton A, Heidenreich PA. A Validated Risk Model for 30-Day Readmission for Heart Failure. Stud Health Technol Inform 2017;245:506-510.
40. Hutt E, Frederickson E, Ecord M, Kramer AM. Associations among processes and outcomes of care for Medicare nursing home residents with acute heart failure. J Am Med Dir Assoc 2003;4:195-199.
41. Medicare Payment Advisory Commission. Report to the Congress: promoting greater efficiency in Medicare. 2007.
42. Konstam MA, Upshaw J. Sisyphus and 30-Day Heart Failure Readmissions: Futility in Predicting a Flawed Outcome Metric. JACC Heart Fail 2016;4:21-23.
43. Jurgens CY, Goodlin S, Dolansky M, et al. Heart failure management in skilled nursing facilities: a scientific statement from the American Heart Association and the Heart Failure Society of America. Circ Heart Fail 2015;8:655-687.