H.H. Keller1, C.M. Steele2, C. Lengyel3, N. Carrier4, S.E. Slaughter5, J.M. Morrison6, L.M. Duizer7
1. Schlegel-University of Waterloo Research Institute for Aging, Waterloo, Canada; 2. Toronto Rehabilitation Institute, University Health Network, Toronto, Canada; 3. Faculty of Agricultural and Food Sciences, University of Manitoba, Winnipeg, Canada; 4. École des sciences des aliments, de nutrition et d’études familiales, Faculté des sciences de la santé et des services communautaires, Université de Moncton, Moncton, Canada; 5. Faculty of Nursing, University of Alberta, Edmonton, Canada; 6. Department of Kinesiology, University of Waterloo, Waterloo, Canada; 7. Department of Food Science, University of Guelph, Guelph, Canada. Corresponding author: Lisa M Duizer, Department of Food Science, University of Guelph, Guelph, ON Canada, N1G 2W1, Phone: 519-824-4120 ext 53410 Email: lduizer@uoguelph.ca
Abstract
Abstract: The aim of this research was to examine the prevalence of olfactory impairment in a sample of individuals living in long term care (LTC) homes and to examine associations between olfactory scores and food and fluid intake. Data were collected as part of a cross-sectional study conducted in 32 LTC homes across 4 provinces in Canada. Olfactory capabilities of 300 individuals were estimated using Burghart Sniffin’ Sticks. Food and fluid intake and self-reported olfactory capabilities were also collected. Based on Sniffin’ Stick scores, participants were classified into groups (anosmic vs not anosmic) with the majority (n=273) classified as anosmic. Differences in dietary and body weight data between the two groups were examined using pooled t-tests. No differences existed between olfactory group and body weight, caloric intake, nutrient intake or overall diet quality. Results indicate that older adults in LTC homes have significant olfactory impairments that do not show an association with food and fluid intake.
Key words: Olfactory capabilities, dietary intake, Making the Most of Mealtimes.
Introduction
Olfactory impairment can have a significant impact on many aspects of an individual’s life, particularly as it relates to nutrition. Individuals with olfactory impairment show an increased risk of poor diet quality (1). Community dwelling women aged 65 who have olfactory losses consume more sweet foods and have a lower preference for fruits and vegetables (2). Recent research however, shows no association between olfactory function and nutritional status when examined using the short and long forms of the Mini Nutritional Assessment (MNA) (3, 4). The MNA tool detects risk of malnutrition through a collation of anthropometric measurements, mobility, food intake and body mass index (5). This tool, however, does not specifically measure food and fluid intake. In one of the few studies where actual nutrient intakes were assessed, no effect of olfactory impairment on macronutrient intake of older Korean adults was found (6). Olfactory impairments, however, were self-reported and not measured using a validated tool. The aim of the current research is to examine the prevalence of olfactory impairment in a sample of older adults living in Canadian long term care (LTC) homes and the associations between olfactory scores when measured using a validated olfaction tool and nutrient and energy intake collected using measures of actual food intake.
Methods
Study Design
Data analysed in this paper were resident level factors that were collected as part of the Making the Most of Mealtimes (M3) study, a cross-sectional research project conducted in 32 LTC homes in 4 Canadian provinces (7). The overall aim of the larger study was to identify and measure multi-level determinants (resident-, dining room- and home-level) of food and fluid intake in residents. The complete protocol of the data collection at all levels of the study has been outlined elsewhere (7). The study was approved by research ethics boards from the participating Universities within the four provinces (University of Waterloo, University of Alberta, University of Manitoba, Université de Moncton, University Hospital Network at the University of Toronto and University of Guelph) and from LTC home sites as required.
Participants
In each of the 32 homes, residents were randomly selected to take part in the study based on the following inclusion criteria: over the age of 65, informed consent provided by the resident or substitute decision maker, no hospital admission in the previous month, residing in the home for at least one month, consumption of an oral diet, and meals consumed in the dining room. Recruitment of residents occurred until a quota of 20 residents per home was met. In total, 639 participated in the study and of these 300 individuals took part in the olfactory tests. These individuals had the cognitive capabilities (CPS score <3) and consented to be involved in olfactory testing.
Resident Level Measures
To measure olfactory capability, participants (n=300) were presented with the “Sniffin’ Stick” – Screening 12 Test (Burghart Messtechnik GmbH). This measure has high test-retest reliability and has previously been used to characterize individuals based on olfactory capabilities (8). In brief, the “Sniffin’ Stick” test involves presenting participants with a “pen” infused with an odour. To ensure consistency with testing, a trained research assistant removed the lid from the pen and held it approximately 2 cm from each nostril of the participant. After sniffing the pen with each nostril, the participant was asked to identify the odour by pointing to a labelled picture from a choice of four placed in front of them. The research assistant recorded the response prior to moving on to the next pen. In total, 12 pens were presented to participants for sniffing. All participants were allowed to take breaks as necessary. Residents (n=295) also self-reported their olfactory capability by rating their ability to smell food as poor, fair, good or excellent.
Weighed food intake of each participant was collected over three non-consecutive days (2 weekdays and one weekend day) by trained research assistants. Main plate food items were individually weighed before and after each of nine meals and the amount consumed was determined through subtraction. Consumption of beverages, side dishes and snacks was estimated using the production menu and by measuring serving ware. Consumption of food between meals was estimated by observing participants and/or asking residents, family and staff. Food Processor Nutrition Analysis Software version 10.14.1 (Esha Research, Salem, OR, USA) was used for nutrient analysis and estimates of energy (kcal and kcal/body weight), protein (g and g/kg body weight), carbohydrate (g/d) and nutrient intake (Vitamins A, B1, B2, B3, B6 and B12, C, D, E, folate, calcium, copper, iron, magnesium, phosphorus, selenium and zinc). Micronutrient intakes were used to determine nutrient adequacy ratio (NAR) as outlined by Kant (9). For each vitamin, this ratio was calculated as the adjusted intake from food or fluid (no multi-nutrient pills) divided by the recommended dietary allowance (RDA) for the nutrient (by gender and age); a maximum value of 1.0 was used (e.g., intake = RDA). The mean adequacy ratio (MAR) was calculated by averaging the 17 NAR’s. A higher MAR score indicated better diet quality, where a value equal to 1.0 was interpreted as all micronutrients being consumed above the RDA for the resident. Demographic information including age (years), sex (male/female), body weight (kg) and body mass index (BMI), estimated using ulna length, were also collected and used in this analysis.
Statistical Analysis
Number of correct responses obtained for the olfactory test were used to categorize individuals based on their olfactory capabilities: anosmic – 6 or less odours; potentially anosmic – scores of 7 to 10; and normosmic – scores of 11 or 12 (8). Frequency of individuals falling within each category was calculated. The self-reported smell capabilities were then analysed using a one-way analysis of variance (ANOVA) to examine the association between actual smell ability and self-reported olfactory capability.
Given the low number of individuals in the normosmic group (1% of the population), olfactory capabilities were re-categorized into anosmic (those who scored ≤ 8 on the olfactory test) and not anosmic (those who scored greater than 8) as per Hummel (8). Differences in resident characteristics and dietary intakes were examined between these two groups using pooled t-tests for equal variance.
Results & Discussion
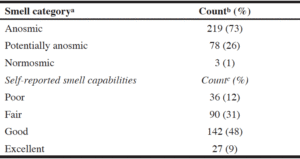
Olfactory categorizations shown in Table 1 indicate that the majority of the LTC home residents (over 70%) completing the olfactory test were classified as anosmic. Counts of responses ranged from 11 individuals not able to correctly identify any smells (score of 0) to one person correctly identifying all samples (score of 12). The median score was 5. Others have found similar prevalence rates; prevalence of olfactory impairment has been shown to increase with age where 62.5% between the ages of 80 and 97 have impaired olfaction (10).
a. Based on categorization by Hummel et al. (8); b. n=300; c. n=295 due to unanswered data
When asked about their olfactory capability, almost 50% of the sample felt that their smell capabilities were “good” (Table 1). Those who self-reported their capabilities to be poor had significantly lower “Sniffin’ Sticks” scores (M = 3.33, SD = 2.42) than those who indicated their smell capability as fair (M = 4.74, SD = 2.39), good (M = 5.58, SD = 2.38) or excellent (M = 5.11, SD = 2.48; F3,291 = 8.88, p < 0.0001) indicating that individuals are aware that they have olfactory losses.
When individuals were reclassified into two groups (anosmic and not anosmic) and groups compared, no differences were found between olfactory ability and body weight, BMI, or any of the dietary intake measures (Table 2). This result confirms previous evidence on lack of an association between olfactory ability when other olfactory measures are used and dietary intake (6, 11).
Given that the smell of a food typically contributes to a desire to consume a food, the results observed in this study may appear counterintuitive. There are, however, a number of reasons why a reduction in olfactory ability does not affect food intake. First, it is well documented that changes to olfactory capabilities are gradual and not easily noticed by individuals (12). This may be one reason why others have not found a relation between olfactory dysfunction and preference for flavor enhanced foods (13, 14). Second, there are other factors, aside from the odours and flavours associated with food that contribute to the desire to eat. The first activity that individuals undergo when food is put in front of them is to look at the food. It is at this point that a judgement is made as to whether or not the food will be consumed. While there is evidence that individuals who consume modified textured diets use the appearance of the food as one indicator to decide if the food is safe for them to eat (15), whether this holds true for individuals consuming a regular textured diet has not been clearly elucidated. Future research should assess the impact of appearance on the acceptability of foods served to individuals in LTC. It may be that by making a food look more appealing, individuals may be more likely to eat it regardless of their olfactory capabilities. Last, for individuals in LTC, mealtime is an important part of the day. While food is essential to mealtimes, the larger context of the dining experience, including interactions with others is also important. Recent research by Trinca et al (16) showed that energy and protein intakes were greater when family/volunteers were present at the meal. It may be that social factors such as this compensate for any olfactory impairments present in the population and may be more relevant to support intake.

Table 2
Resident characteristics (demographics and dietary intakes) based on likelihood of olfactory impairment
a. Differences not examined; b. Mean adequacy ratio calculated by averaging nutrient adequacy ratios for 17 vitamins
Although it is often suggested that individuals with olfactory impairments should be provided with foods with enhanced tastes and smells to improve intake, our results suggest that this strategy may not be useful and that factors other than olfactory impairment are contributing to the high levels of inadequate intake within this population.
Funding: Funding for the Making the Most of Mealtimes project was provided by the Canadian Institutes of Health Research (grant numbers 201403MOP-326892-NUT-CENA-25463)
Conflict of interest: Dr. Keller reports grants from Canadian Institutes for Health Research, during the conduct of the study; Dr. Steele reports grants from National Institutes of Health, other from International Dysphagia Diet Standardisation Initiative, outside the submitted work. No other conflicts have been reported.
Ethical standard: Ethics approval has been received from the University of Waterloo, University of Alberta, University of Manitoba, Université de Moncton, University Hospital Network at the University of Toronto and University of Guelph and from LTC home sites as required.
References
1. Gopinath B, Russell J, Sue CM, Flood VM, Burlutsky G, Mitchell P. Olfactory impairment in older adults is associated with poorer diet quality over 5 years. Eur J Nutr. 2016;55(3):1081-1087. doi:10.1007/s00394-015-0921-2.
2. Duffy VB, Backstrand JR, Ferris AM. Olfactory dysfunction and related nutritional risk in free-living, elderly women. J Am Diet Assoc. 1995;95:879-884.
3. Smoliner C, Fischedick A, Sieber CC, Wirth R. Olfactory function and malnutrition in geriatric patients. J Gerontol A Biol Sci Med Sci. 2013;68(12):1582-1588. doi:10.1093/gerona/glt085.
4. Toussaint N, de Roon M, van Campen JPCM, Kremer S, Boesveldt S. Loss of olfactory function and nutritional status in vital older adults and geriatric patients. Chem Senses. 2015;40(3):197-203. doi:10.1093/chemse/bju113.
5. Guigoz Y. The mini-nutritional assessment (MNA(R)) review of the literature – What does it tell us? J Nutr Health Aging. 2006;10:466-487.
6. Kong IG, Kim SY, Kim MS, Park B, Kim JH, Choi HG. Olfactory dysfunction is associated with the intake of macronutrients in Korean adults. PLoS One. 2016;11(10):1-10. doi:10.1371/journal.pone.0164495.
7. Keller HH, Carrier N, Slaughter S, et al. Making the Most of Mealtimes (M3): protocol of a multi-centre cross-sectional study of food intake and its determinants in older adults living in long term care homes. BMC Geriatr. 2017;17(1):15. doi:10.1186/s12877-016-0401-4.
8. Hummel T, Konnerth CG, Rosenheim K, Kobal G. Screening of olfactory function using a 4 minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol. 2001;110:976-981.
9. Kant A. Indexes of overall diet quality: A review. J Am Diet Assoc. 1996;96(8):785-791.
10. Murphy C, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Prevalence of Olfactory Impairment. J Am Med Assoc. 2002;288(18):2307-2312.
11. Ferris AM, Duffy VB. Effect of Olfactory Deficits on Nutritional Status: Does Age Predict Persons at Risk? Ann N Y Acad Sci. 1989;561(1):113-123. doi:10.1111/j.1749-6632.1989.tb20975.x.
12. Wysocki CJ, Pelchat ML. The effects of aging on the human sense of smell and its relationship to food choice. Crit Rev Food Sci Nutr. 1993;33(1):62-82. doi: 10.1080/10408399309527613.
13. Forde C, Delahunty C. Understanding the role cross-modal sensory interactions play in food acceptability in younger and older consumers. Food Qual Prefer. 2004;15(7-8):715-727.
14.. Kremer S, Bult, JHF, Mojet J, Kroeze JHA. Compensation for age-associated chemosensory losses and its effect on the pleasantness of a custard dessert and a tomato drink. Appetite. 2007;41(1):96-103.
15. Keller HH, Duizer LM. What do consumers think of pureed food? Making the most of the indistinguishable food. J Nutr Gerontol Geriat. 2014;33(3): 139-159.
16. Trinca V, Morrison J, Slaughter S, Keller H. Making the Most of Mealtimes (M3): effect of eating occasions and other covariates on energy and protein intake among Canadian older adult residents in long-term care. J Hum Nutr Diet. 2019. Doi: 10.1111/jhn.12686. [Epub ahead of print].