A.L. Hume1,2, S. Osundolire2, A.K. Mbrah2, A.P. Nunes2, K.L. Lapane2
1. College of Pharmacy, University of Rhode Island, Kingston, RI, USA; 2. Division of Epidemiology, Department of Population and Quantitative Health Sciences, University of Massachusetts Medical School, Worcester, MA, USA
Corresponding author: Kate L. Lapane, PhD, University of Massachusetts Medical School, Worcester, MA, Email: kate.lapane@umassmed.edu, Telephone: 508-856-8798, Fax:508-856-8993
Jour Nursing Home Res 2022;8:10-19
Published online May 12, 2022, http://dx.doi.org/10.14283/jnhrs.2022.4
Abstract
Background: About 29.2% of American adults ≥ 65 years of age have diabetes mellitus, but details regarding diabetes management especially among nursing home residents are dated. Objectives: Evaluate the prevalence of antihyperglycemic agents in residents with diabetes mellitus and describe resident characteristics using major drug classes. Design: Cross-sectional study. Setting: Virtually all United States nursing homes. Participants: 141,636 residents with diabetes mellitus. Measurements: Minimum Data Set (2016) and Medicare Part D claims determined use of metformin, sulfonylureas, meglitinide analogs, alpha-glucosidase inhibitors, TZDs, DPP4 inhibitors, SGLT2 inhibitors, GLP1 agonists, as monotherapy and with basal insulin. Results: Seventy-two percent received antihyperglycemic drugs [most common: basal insulins (53.9% total; 46.9% with other non-insulin agents), metformin (35.5% total; 14.2% monotherapy), sulfonylureas (19.6% total; 6.3% monotherapy), and DPP4 inhibitors (12.2% total; 2.2% monotherapy)]. Sixty-three percent of meglitinide monotherapy versus 34.1% of metformin monotherapy users; and 38.3% meglitinide–basal insulin versus 22.2% metformin–basal insulin users were ≥85 years. Obesity was greater among users of GLP1 agonists compared to those receiving other agents (monotherapy: 60.5% versus 33-42%; with basal insulin: 76.2% versus 50-58%). End-stage renal disease was least prevalent among metformin users (monotherapy: 6.6%; with basal insulin: 8.8%) and most common among meglitinide monotherapy (19.6%) and GLP1 agonists with basal insulin (22%) users. Conclusions: There is heterogeneity of diabetes treatment in nursing homes. Use of antihyperglycemic drugs with a higher risk of hypoglycemia, such as insulin with sulfonylureas or meglitinides, continue in nursing home residents.
Key words: diabetes, nursing homes, antihyperglycemic medications, insulin.
Introduction
An estimated 537 million adults are living with diabetes globally (1). In 2019, over 37 million American adults have diabetes with 29.2% of individuals ≥ 65 years of age having the disease, either diagnosed or undiagnosed (2). National data on the prevalence of diabetes among residents of American nursing homes are limited and dated. A sample of almost 12,000 individuals from the 2004 U.S. National Nursing Home Survey indicated an overall prevalence of 24.5%, which was markedly higher among nonwhites (3).
The optimal management of diabetes differs in older adults versus younger individuals, especially in the nursing home setting (4-6). The presence of multimorbidity and cognitive impairment, dependence in instrumental activities of daily living, overall frailty, and risk of hypoglycemia influence the treatment goals (4, 5). The American Geriatric Society (AGS) Choosing Wisely guide recommends a hemoglobin (Hb)A1c goal between 8 and 9 for older adults with multiple comorbidities and a limited life expectancy (7). The American Diabetes Association (ADA) standards emphasize that for patients with poor health, including those in nursing homes, treatment decisions should be based on avoiding hypoglycemia as well as symptomatic hyperglycemia (5, 6).
A greater focus on the general levels of frailty among older individuals with diabetes have emerged recently (8-10). Healthy, prefrail, or mildly frail older diabetics with a life expectancy over 10 years may have an HbA1c target less than 7.5% with re-evaluation of long-acting sulfonylureas and insulin, as well as changes in renal function (8-10). Individuals with moderate to severe frailty and a reduced life expectancy have an HbA1c target less than 8% with the emphasis on preventing functional and cognitive decline. General avoidance of sulfonylureas, as well as renally dosing antihyperglycemic drugs, are among the key treatment strategies. For older adults with severe frailty and a marked reduction in life expectancy, an HbA1c target of less than 8.5% may be more appropriate, while balancing the risk of hypoglycemia and resulting falls and hospitalizations, with the risk of infections and urinary incontinence from hyperglycemia (9, 10).
The clinical trial evidence base for treating diabetes in frail older adults remains limited. A 2016 review of four major randomized controlled trials identified that intensive glycemic management did not improve microvascular or macrovascular outcomes in older adults for at least the first 8 to 10 years, respectively, and individuals over 80 years of age were excluded (11). The risk of hypoglycemia was estimated to be increased between 1.5- and 3-fold which would likely be increased in a frail older population (11).
Treatment of older adults with diabetes is focused on using drugs with a low risk of hypoglycemia, avoidance of other adverse effects, and general overtreatment (5, 6, 10). Metformin has traditionally been the first-line therapy for type 2 diabetes because of its low risk of hypoglycemia when used as monotherapy as well as its cardiovascular benefits (5, 10). However, adverse effects including loss of appetite, diarrhea, vitamin B12 deficiency, and potential lactic acidosis may limit its use among frail older adults (5, 10).
Newer drugs including dipeptidyl peptidase-4 (DPP4) inhibitors, glucagon-like peptide-1 (GLP1) agonists, and sodium glucose co-transporter 2 (SGLT2) inhibitors might offer advantages in older adults with diabetes due to their low risk of hypoglycemia. Overall, the evidence base on the safety and efficacy of these drugs in frail older diabetics is limited. For the SGLT2 inhibitors, adverse effects such as volume depletion, urinary tract infections, candidiasis, and euglycemic diabetic ketoacidosis limit their use in frail older adults (5, 10).
Although the challenges in managing diabetes in frail older adults are widely acknowledged, data on the use of newer antihyperglycemic agents in this population remains limited. Using national data from 2016, this study identified the prevalence and characteristics of use of major antihyperglycemic drug classes, either as monotherapy or in combination with basal insulin, in nursing home residents.
Methods
The Institutional Review Board of the University of Massachusetts Medical School approved this study.
Sample
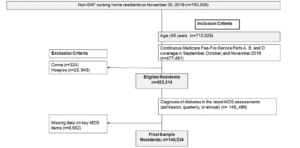
This study linked the Minimum Data Set Version 3.0 (MDS 3.0, 2016), Medicare Master Beneficiary Summary File, and Medicare claims (Part A and D). MDS 3.0 is a standardized tool of over 400 items to assess residents’ health including medical conditions, cognitive and physical functioning, pain, and mood, among other measures (12). The MDS 3.0 measures have demonstrated validity and reliability (13). Medicare is a United States federal health insurance program for individuals aged ≥ 65 years and is comprised Part A (hospitalizations, skilled nursing care and hospice care), Part B (office visits, selected outpatient services), and Part D (prescription drugs). Medicare recipients choose either Medicare Fee-for-Service or Medicare Advantage plans. Medicare beneficiaries selecting Medicare Advantage must use health care providers who participate in the plan’s network, whereas those who elect Medicare Fee-for-Service do not have those restrictions. The MDS is federally mandated for the clinical assessment of all residents in all Medicare/Medicaid certified nursing homes. MDS assessments are conducted by trained nursing home staff on all residents at admission, quarterly, annually, when significant changes in status occur, and at discharge (14). Figure 1 shows the sample construction. The final sample included 141,636 residents.
Medications
We used Medicare Part D claims to identify antihyperglycemic use. Medicare Part D is the prescription drug program available to older adults eligible for Medicare. For all drugs except insulin, coverage was present if the prescription refill date and number of days’ supply included the date of the MDS assessment. The oral antihyperglycemic drugs included metformin, sulfonylureas, meglitinide analogs, alpha-glucosidase inhibitors, thiazolidinediones (TZDs), DPP 4inhibitors, and SGLT2 inhibitors. Injectable drugs included the GLP1 agonists and insulins. Because the days’ supply variable for insulin may not be useful to determine whether or not insulin was used on a particular day, we considered the resident an insulin user based on the Part D claims in the 90 days preceding the MDS annual assessment. The insulins were classified as basal (degludec, detemir, or glargine), regular/rapid (aspart, glulisine, lispro, regular), or miscellaneous (aspart protamine/aspart, lispro protamine/ lispro, neutral protamine hagedorn (NPH). We considered drugs to be monotherapy if only one agent covered the index date. We also identified residents who were prescribed combinations of basal insulin and metformin, sulfonylureas, meglitinide analogs, DPP4 inhibitors, TZDs, or GLP1 agonists. In this study, we chose to focus on residents who were receiving treatment either with monotherapy or one drug in combination with an insulin. Although combination oral antihyperglycemic therapy is common in independently living older adults, few nursing home residents were taking multiple oral drugs. We also identified residents receiving angiotensin converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or statins.
Measures
We categorized the level of frailty as non-frail (0 – 5), pre-frail (6 – 7), and frail (≥8) (12, 15). The MDS Activities of Daily Living (ADL) Hierarchy Scale ranges from 0 – 2 (no/mild limitations), 3 – 4 (ADL limitations), and 5 – 6 (dependence) (16). The Brief Interview for Mental Status (17) and the Cognitive Performance Scale (18) were combined to form the Cognitive Function Scale (19) ( no/mild, moderate, and severe impairment).
We used the MDS Agitated and Reactive Behavior Scale to characterize behavioral issues (20). We used the validated MDS Confusion Assessment Method (CAM) scale to define delirium and combined those with probable and possible delirium (21, 22).
Comorbid Conditions
Most diabetic complications and comorbid conditions were identified using a look-back period of 7 days from the date of the assessment. Licensed clinicians, including physicians, nurse practitioners, physician assistants, clinical nurse specialists or other authorized staff as permitted by state law, provided documentation in support of comorbid conditions and diabetic complications in the medical records. With the absence of an active diagnosis in the progress notes, a recent onset or exacerbation of the condition, confirmed by a positive study, test or procedure, or recent hospitalization of the resident with acute symptoms or change in ongoing medication/therapy were also indications of an active disease (14). Conditions of interest included diagnoses of depression, Alzheimer’s disease/dementia, hypertension, heart failure, coronary artery disease, stroke, and renal insufficiency/end-stage renal disease (ESRD).
Foot ulcers were determined by a 7-day look-back period of documentation in the medical record confirming that a resident with diabetes mellitus has an ulcer on the plantar surface of the foot closer to the metatarsal (23). Amputation was determined using MDS items identifying residents currently receiving amputation/prosthesis care for 15 or more minutes for at least a day in the past 7 days (14). Urinary tract infections had a look-back period of 30 days showing specific documentation in the medical records of an active diagnosis (12). Urinary incontinence was categorized by severity level ranging from always continent, occasionally incontinent (1–6 episodes of urinary incontinence), frequently or always incontinent (≥7 episodes of urinary incontinence), or use of catheter based on a 7-day look-back period in the progress notes (12). Residents’ body mass index (BMI) was categorized as <18.5, 18.5 to <25, 25 to <30, 30 to < 40, ≥40 kg/m2 (11). Falls were identified if the resident fell anytime in the past 6 months prior to admission or if the resident sustained a fall at least once since admission or the prior assessment. Pain was evaluated using a look-back period of 5 days. Residents self-reported pain and if self-report was not possible, staff documented presence of pain.
Analyses
We described the sample by estimating the prevalence of sociodemographic, clinical characteristics, and comorbid conditions. We described the patterns of antihyperglycemic prescriptions by reporting the nine major classes of drugs, as monotherapy and in combinations with basal insulin. We examined the prevalence of various resident sociodemographic characteristics, functional impairment, cognitive impairment, and clinical comorbidities by selected drugs. Because the sample size is large and trivial differences were statistically significant, we have not used p-values in the text. We considered a 5% or greater absolute difference in prevalence between subgroups to be noteworthy.
Results
Overall, 33.3% of the 214,000 had diabetes, of whom 68.5% were women, 18.1% were Black, and 6.9% Hispanic. Thirty-five percent were aged 75-84 years and 38% were aged ≥85 years. Twenty-four percent were pre-frail and 58.6% were frail. Twenty-four percent were dependent in ADLs and 42.4% had moderate to severe cognitive impairment. Comorbid conditions included hypertension (87.8%), hyperlipidemia (58.9%), depression (56.3%), dementia (57.9%), heart failure (26.9%), and chronic kidney disease/ESRD (17.5%). Forty-one percent had BMI ≥ 30kg/m2.
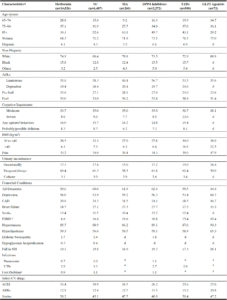
Table 1 shows that 39% of pre-frail and 39.7% of frail residents were ≥85 years. While 37.9% of frail residents were dependent in ADLs, 1.3% of non-frail residents were dependent in ADLs. With respect to cognitive function, 15.5% of frail residents and 0.9% of non-frail residents had severe impairment. Obesity was common, regardless of frailty status. Comorbid conditions were similar across frailty status with few exceptions, including dementia (62.5% among frail residents, 47.9% among non-frail residents) and stroke (17.4% of frail residents and 7.7% of non-frail residents). Fall histories were more common among pre-frail residents (23.0%) than among frail (16.0%) and non-frail residents (17.4%). Statin use was common, with 44.1% of frail residents and 53.4% of non-frail residents using statins. Use of an ACEI or ARB was present in over a third of frail diabetic residents.
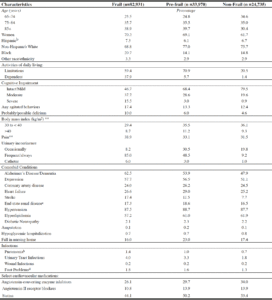
Table 1
Characteristics of Long-stay Residentsa with Diabetes Mellitus living in US nursing homes in 2016, stratified by level of frailty (N=141,636)
a. Long-stay defined as residents with an annual assessment in 2016; b .Missing data for race/ethnicity (n=2,200), pneumonia (n=20), pain (n=3,165), and body mass index (n=2,090); c. End stage renal disease includes renal insufficiency or renal failure; d. Foot problems include infection, diabetic foot ulcers, and other open lesion(s) of the foot.
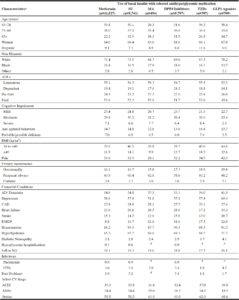
Seventy-two percent received antihyperglycemics (Table 1). The most commonly used agents were basal insulins (53.9% of the 102,451 total treated, 46.9% with other non-insulin agents), metformin (35.5% of total treated; 14.2% monotherapy), sulfonylureas (19.6% of total treated; 6.3% as monotherapy), and DPP4 inhibitors (12.2% of total treated; 2.2% monotherapy). Few residents were taking one of the three newer antihyperglycemic classes as monotherapy. The sulfonylureas included glipizide (63.6%, n=12,757), glimepiride (33.6%, n=6,745), and glyburide (2.2%, n=436). We are unable to report the exact number of chlorpropamide users per our data use agreement because the number is less than 11. Thirty-six residents took multiple sulfonylureas including 24 receiving glipizide and glimepiride. Sulfonylurea combinations were used by 154 residents; 48 received glyburide/metformin and 106 received glipizide/ metformin. Meglitinide analogs were used by 1.1% of treated residents (0.3% as monotherapy, 43.4% (494/1,138) with basal insulin); 42.0% on nateglinide and 57.0% on repaglinide. Of the 1,018 residents taking a GLP1 agonist, liraglutide was prescribed to 67.3%, followed by 25.3% for exenatide, and 7.8% for dulaglutide. The primary DPP4 inhibitors were sitagliptin (68.3%) and linagliptin (30.1%). Of the 569 residents taking an SGLT2 inhibitor, canagliflozin comprised 81%. Pioglitazone was the only TZD used.
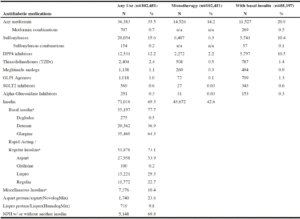
Table 2
Antihyperglycemic use (any use, monotherapy, with basal insulin) among nursing home residents with diabetes treated
with antidiabetic medications (n=102,451)
a. May not total to 100% as multiple agents may have been used in the 90 days preceding the MDS annual assessment
Claims for insulin within the 90 days of the MDS assessment date were available for 69.3% of treated residents. Among those on insulin, claims for basal insulin were present for 77.7% (glargine: 64.3% of basal insulin use; detemir: 36.9%; degludec: 0.5%). These percentages exceed 100 due to residents having multiple claims for different basal insulins. NPH insulin alone or in combination with other insulin products was used in 5,148 residents. Rapid/short-acting insulin usage was present in 73.1% of treated residents, of which 53.9% were insulin aspart users, 29.3% were on insulin lispro, 22.7% were on regular insulin, and 0.2% were on glulisine. Among residents receiving a basal insulin, 70.3% also received a rapid/short acting insulin.
Tables 3 and 4 show that among those receiving monotherapy or basal insulin with selected antihyperglycemics, the age distribution varied. For example, 63.1% of meglitinide monotherapy users were ≥85 years versus 34.1% of metformin monotherapy users; when combined with basal insulin, 38.3% meglitinide versus 22.2% metformin users were ≥85 years. Frailty was similar across the antihyperglycemic groups. Most residents were frequently or always incontinent, regardless of antihyperglycemic regimen. At least a third had pain documented on their annual assessment. A history of falls was present in almost 20% of residents across all treatment categories as shown in Tables 3 and 4. The proportion of obese residents was greater among GLP1 agonist users compared to those receiving other agents (monotherapy: 60.5% versus 33-42%; with basal insulin: 76.2% versus 50-58%). Comorbid conditions including dementia, depression, and cardiovascular diseases were highly prevalent. The presence of ESRD was the least common among metformin users (monotherapy: 6.6%, with basal insulin: 8.8%) and most common among meglitinide monotherapy users (19.6%) and GLP1 agonists with basal insulin: 22%).
a. Missing data– for metformin group: Body mass index (n=190), pain (n=301), race/ethnicity (n=234) and pneumonia (n=1); for the sulfonylurea group: Body mass index (n=81), pain (n=137), and race/ethnicity (n=90); for the meglitinide group: Body mass index (n=4), pain (n=7), and race/ethnicity (n=2); for the DPP4 Inhibitors group: Body mass index (n=23), pain (n=49), and race/ethnicity (n=33); for the thiazolidinedione group: Body mass index (n=4), pain (n=13), and race/ethnicity (n=10); for the GLP1 Agonist group: Body mass index (n=1) and pain (n=1); SU = Sulfonylureas, MA = Meglitinide analogs, TZD = Thiazolidinediones, ADLs= Activities of daily living, BMI = Body mass index, AD = Alzheimer’s disease, CAD = Coronary artery disease, ESRD = End stage renal disease, UTI = Urinary tract infections, ACEI = Angiotensin converting enzyme inhibitors, ARBs = Angiotensin II receptor blockers; b. ESRD includes renal insufficiency or renal failure; c. Foot Problem include infection, diabetic foot ulcers, and other open lesion(s) of the foot; d. Per Data Use Agreement, cell sizes less than 11 cannot be shown.
a. Missing data– for metformin group: Body mass index (n=133), pain (n=171), race/ethnicity (n=175) and pneumonia (n=2); for the sulfonylurea group: Body mass index (n=62), pain (n=97), pneumonia (n=1), and race/ethnicity (n=84); for the meglitinide group: Body mass index (n=7), pain (n=7), and race/ethnicity (n=8); for the DPP4 Inhibitors group: Body mass index (n=64), pain (n=88), pneumonia (n=1), and race/ethnicity (n=82); for the TZD group: Body mass index (n=10), pain (n=14), and race/ethnicity (n=11); for the GLP1 Agonist group: Body mass index (n=4), pain (n=17), and race/ethnicity (n=7); SU = sulfonylureas, MA = Meglitinide analogs, TZD = thiazolidinediones, ADLs= Activities of daily living, BMI = Body mass index, AD = Alzheimer’s disease, CAD = Coronary artery disease, ESRD = End stage renal disease, UTI = Urinary tract infections, ACEI = Angiotensin converting enzyme inhibitors, ARBs = Angiotensin II receptor blockers; b. End stage renal disease includes renal insufficiency or renal failure; c. Foot Problems include infection, diabetic foot ulcers, and other open lesion(s) of the foot; d. Per Data Use Agreement, cell sizes less than 11 cannot be shown.
Discussion
In our study of nursing home residents with diabetes, over 82% of residents were frail or prefrail and 38% were 85 years of age or older. Twenty-eight percent were not receiving drug therapy, although they may have been managed by nonpharmacologic approaches. In a 2001 study, we identified that 47% of residents with diabetes did not receive antihyperglycemic therapy (23).
This study identified multiple potentially inappropriate prescribing patterns, primarily related to sulfonylureas. Most problematically, the use of sulfonylureas has continued both as monotherapy and in combination with basal insulin regimens. Sulfonylureas are well recognized to increase the risk of severe and prolonged hypoglycemia in frail older diabetics (24, 25). In 2020 cohort study involving over 200,000 adults with diabetes, age of 75 years, multimorbidity, and use of sulfonylureas and/or insulin significantly increased the risk of hypoglycemia-related emergency department (ED) visits and hospitalizations (26). The specific risk of these events was 6.7-fold higher with sulfonylureas, 12.5-fold higher with basal insulins, 13.8-fold higher with basal insulin combined with sulfonylurea use, 23.2-fold higher with bolus insulins, and 27.7-fold higher with basal-bolus regimens (26). Importantly, this large study was of independently living adults with diabetes, where frail nursing home residents may be at even greater risk of hypoglycemia-related ED visits and hospitalizations.
In addition, significant usage of sulfonylureas was identified in the present study among frail and cognitively impaired older diabetics including those with moderate cognitive impairment. Neurologic manifestations of hypoglycemia such as dizziness may be more common in older diabetics instead of the traditional signs such as tachycardia [25]. Among cognitively impaired nursing home residents, symptoms may present as agitation and thus be easily overlooked as a coexisting condition (25). Fortunately, usage of sulfonylureas was low among those with severe cognitive impairment. In contrast to a Canadian study of 15,034 nursing home residents with diabetes, only 6.6% of residents with severe cognitive impairment in our study were taking sulfonylureas compared with 18.1% in the Canadian study (27). Overall, sulfonylureas may have been continued largely on the basis of their long-term use in residents. Efforts to de-intensify (deprescribe) the treatment of diabetes among frail older adults should focus on the continued use of sulfonylureas.
In our study, metformin alone or in combination with basal insulin, was the most commonly used antihyperglycemic agent, potentially as a result of residents having taken the drug before admission to the nursing home. While the exact dosages of metformin could not be assessed in this study, metformin users had the lowest prevalence of chronic kidney disease/ESRD, consistent with treatment guidelines (5, 6, 10). Although its potential adverse effects on appetite and weight are important in frail residents, concern about the risk for lactic acidosis in the presence of chronic kidney disease and other comorbid conditions has continued among clinicians. A systematic review identified limited evidence of this link when the glomerular filtration rate (GFR) is >30ml/min (28).
Usage of DPP4 inhibitors, SGLT2 inhibitors, and GLP1 agonists was identified in our study, with DPP4 inhibitors, either as monotherapy or combined with a basal insulin, most commonly used of these newer classes. A significant percentage of DPP4 inhibitor users were ≥ 85 years. This finding is consistent with treatment guidelines for managing HbA1c above 8.5% in severely frail older diabetics and among those who need to discontinue other oral agents due to worsening comorbid conditions (8, 10). A retrospective cohort study of long-stay nursing residents demonstrated a 43% reduction in the 1-year risk of severe hypoglycemia among new users of DPP4 inhibitors versus new users of sulfonylureas (29). Rates of severe hyperglycemic events, heart failure, and death were similar between the two groups (29). Although DPP4 inhibitors possess a low risk of severe hypoglycemia, they have been associated with severe, disabling arthralgias which may be overlooked as an adverse drug effect in residents with multimorbidity (30). In our study, few older adults were taking an SGLT2 inhibitor and may be due to risks of volume depletion, urinary tract infections and their cost.
Clinical practice guidelines continue to emphasize reducing the risk of hypoglycemia and symptomatic hyperglycemia in frail older diabetics (5, 6, 10) and consideration of deprescribing. In our study, we could not evaluate the intensity of HbA1c control among the nursing home residents. A 2015 report using data on noninstitutionalized older adults from National Health and Nutrition Examination Survey indicated that the attainment of HbA1c values less than 7% or use of sulfonylureas and insulin was similar between 2001 and 2010, regardless of the complexity of older adults’ health status (31). The Canadian nursing home study reported that residents with severe cognitive impairment were more likely to be treated to intensive HbA1c targets than those with mild/moderate impairment (27). Forty percent of older veterans with diabetes with life limiting illness were potentially overtreated (32).
This study included virtually every nursing home resident with diabetes in the United States and was able to provide detailed information on comorbid conditions and concomitant drug therapies. As a result, the study was able to evaluate cognitive impairment, measures of frailty, and limitations in activities of daily living, which are measures typically unavailable in claims databases. Most importantly, the primary study limitation was a lack of access to HbA1c measurements which would have provided information on the intensity of diabetic control in this vulnerable population. In addition, we did not know the type or duration of the residents’ diabetes, although they likely had longstanding type 2 diabetes.
Conclusion
The management of diabetes in long-stay nursing home residents is challenging and focused on minimizing hypoglycemia and symptomatic hyperglycemia. While metformin either as monotherapy or in combination with basal insulin remains common, the combined use of basal insulins with sulfonylureas or meglitinides may potentially increase the risk of hypoglycemia. DPP4 inhibitors offer a low risk of hypoglycemia and have been increasingly prescribed in this vulnerable population. Despite the availability of drugs that reduce the risk of hypoglycemia, use of higher risk antihyperglycemics continues in nursing home residents with diabetes.
Funding Sources: This study was funded by grants from National Institute of Health Grant/Award Numbers: TL1TR001454 (Dr. Mbrah), R01NR016977 (Dr. Lapane), R21NR019160 (Dr. Nunes), and T32HL120823 (Dr. Osundolire). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Acknowledgements: None
Ethical Approval: This study used routinely- collected administrative and claims dataset and was approved by the University of Massachusetts Medical School Institutional Review Board (protocol number H00019897).
Conflict of Interest: Drs. Hume, Osundolire, Mbrah, Nunes, and Lapane declare that they have no conflict of interest including financial, personal or potential and have nothing otherwise to disclose.
Funding Information: National Institute of Health Grant/Award Numbers: TL1TR001454 (Dr. Lapane), R01NR016977 (Dr. Lapane), and R21NR019160 (Dr. Nunes).
Declaration of Interest: Anne Hume has no conflicts of interest to declare. Seun Osundolire has no conflicts of interest to declare. Attah K. Mbrah has no conflicts of interest to declare. Anthony Nunes has no conflicts of interest to declare. Kate Lapane has no conflicts of interest to declare.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. International Diabetes Federation. IDF Diabetes Atlas, 10th edn. Brussels, Belgium: 2021. Available at: https://www.diabetesatlas.org. Accessed April 22, 2022.
2. Statistics on Diabetes. American Diabetes Association (online). Available at https://www.diabetes.org/resources/statistics/statistics-about-diabetes. Accessed April 22, 2022.
3. Resnick HE, Heineman J, Stone R, Shorr RI. Diabetes in U.S. nursing homes, 2004. Diabetes Care 2008;31(2):287-288.
4. LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2019;104(5):1520–1574
5. Munshi MN, Florez H, Huang ES, et al. Management of diabetes in long-term care and skilled nursing facilities: A position statement of the American Diabetes Association. Diabetes Care 2016;39:308-318.
6. American Diabetes Association. 12. Older adults: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021;44(Suppl 1):S168-S179.
7. Ten things clinicians and patients should question. American Geriatrics Society. Available at: https://www.choosingwisely.org/societies/american-geriatrics-society/ Accessed August 6, 2021.
8. Sinclair AJ, Abdelhafiz A, Dunning T, et al. An international position statement on the management of frailty in diabetes mellitus: Summary of recommendations. J Frailty Aging 2018; 7:10-20.
9. Strain WD, Hope SV, Green A, et al. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med 2018;35:838-845.
10. Strain WD, Down S, Brown P, Puttanna A, Sinclair A. Diabetes and frailty: An expert consensus statement on the management of older adults with type 2 diabetes. Diabetes Ther 2021;12:1227-1247.
11. Lipska KJ, Krumholz H, Soones T, Lee SJ. Polypharmacy in the aging patient: A review of glycemic control in older adults with type 2 diabetes. JAMA 2016; 315(10):1034-1045.
12. Centers for Medicare & Medicaid Services (2016). Long-Term Care Facility Resident Assessment Instrument 3.0 User’s Manual. Department of Health & Human Services.
13. Saliba D, Buchanan J. Development and validation of a revised nursing home assessment tool: MDS 3.0 Rand Corp 2008.
14. Centers for Medicare & Medicaid Services (2019). Medicare Claims Processing Manual. Available at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c11.pdf» https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c11.pdf. Accessed October 9, 2021.
15. Kaehr EW, Malmstrom TK, Morley JE. FRAIL-NH predicts outcomes in long term care. J Nutr Health Aging 2016;20(2):192-198.
16. Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. J Gerontol A Biol Sci Med Sci 1999; 54(11): M546-553.
17. Chodosh J, Edelen MO, Buchanan JL, et al. Nursing home assessment of cognitive impairment: development and testing of a brief instrument of mental status. J Am Geriatr Soc 2008; 56(11): 2069-2075.
18. Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994 Jul;49(4):M174-182. doi: 10.1093/geronj/49.4.m174. PMID: 8014392.
19. Thomas KS, Dosa D, Wysocki A, Mor V. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care 2017; 55(9): e68-e72.
20. McCreedy E, Ogarek JA, Thomas KS, Mor V. The Minimum Data Set Agitated and Reactive Behavior Scale: Measuring Behaviors in Nursing Home Residents with Dementia. J Am Med Dir Assoc. 2019 Dec;20(12):1548-1552. doi: 10.1016/j.jamda.2019.08.030. Epub 2019 Oct 31. PMID: 31678075; PMCID: PMC7008595.
21. Inouye SK, Van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113:941–948.
22. Inouye SK. The Short Confusion Assessment Method (Short CAM): Training Manual and Coding Guide. Boston: Hospital Elder Life Program. 2014 www.hospitalelderlifeprogram.org/uploads/disclaimers/Short_CAM_Training_Manual_10-9-14.pdf).
23. Spooner JJ, Lapane KL, Hume AL, Mor V, Gambassi G. Pharmacologic treatment of diabetes in long-term care. J Clinical Epidemiol 2001;54:525-530.
24. 2019 American Geriatrics Society Beers Criteria Update Panel. American Geriatric Society 2019 updated AGS Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67(4): 674-694.
25. Abdelhafiz AH, Rodriguez-Manas L, Morley JE, Sinclair AJ. Hypoglycemia in older people – a less well recognized risk factor for frailty. Aging Dis 2015; 6:156-167.
26. McCoy RG, Lipska KJ, Van Houten HK, Shah ND. Association of cumulative multimorbidity, glycemic control and medication use with hypoglycemia-related emergency department visits and hospitalizations among adults with diabetes. JAMA. Network Open. 2020;3(1):e1919099. doi:10.1001/jamanetworkopen.2019.19099.
27. Lega IC, Campitelli MA, Matlow J, et al. Glycemic control and use of high-risk antihyperglycemic agents among nursing home residents with diabetes in Ontario, Canada. JAMA Intern Med 2021; doi:10.1001/jamainternmed.2021.0022
28. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: A systematic review. JAMA 2014;312(24):2668-2675.
29. Zullo AR, Smith RJ, Gutman R, et al. Comparative safety of dipeptidyl peptidase-4 inhibitors and sulfonylureas among frail older adults. J Am Geriatr Soc 2021;69:2923-2930.
30. FDA drug safety communication: FDA warns that DPP-4 inhibitors for type 2 diabetes may cause severe joint pain. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-dpp-4-inhibitors-type-2-diabetes-may-cause-severe-joint-pain Accessed 8/10/21.
31. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015; 75(3):356-362.
32. Niznik JD, Hunnicutt JN, Zhao X, et al. Deintensification of diabetes medications among veterans at end of life in VA nursing home. J Am Geriatr Soc 2020;68(4):736-745.