T. Kaipainen1,2, M. Tiihonen1, S. Hartikainen1, I. Nykänen1,3
1. Kuopio Research Centre of Geriatric Care, School of Pharmacy, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland; 2. Institute of Public Health and Clinical Nutrition, Unit of Clinical Nutrition, Faculty of Health Sciences, University of Eastern, Finland, Kuopio, Finland; 3. Research Centre for Comparative Effectiveness and Patient Safety (RECEPS), School of Pharmacy, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland. Corresponding author: Irma Nykänen, Address Kuopio Research Centre of Geriatric Care, Faculty of Health Sciences, University of Eastern Finland, Kuopio Campus, P.O.BOX 1627, FI-70211 Kuopio, Finland, Phone +358 40 355 2991, Fax +358 17 162 131, E-mail Irma.Nykanen@uef.fi
Abstract
Objectives: To describe nutritional status and to detect factors associated with it in home care clients aged 75 years or older. Design: A cross-sectional study with a population-based sample. Setting: Home care.Participants: The study included 267 home care clients in three cities in Finland. Measurements: The outcomes were nutritional status (Mini Nutritional Assessment, MNA), body mass index (BMI), functional ability (Barthel Index, Instrumental Activities of Daily Living, IADL), cognitive function (Mini Mental State Examination, MMSE), depression (Geriatric Depression Scale, GDS-15), comorbidity (Functional Comorbidity Index, FCI), Vitamin D and drug use and levels of blood albumin and haemoglobin. Chewing problems and dry mouth were assessed by using a structured interview. Results: According to the MNA, a majority (86%, n = 229) of all home care clients were at risk of malnutrition or were malnourished. Persons at risk of malnutrition or who were malnourished used more drugs and had a higher depressive score and lower Barthel Index, IADL and MMSE scores than well-nourished participants. Multivariate analysis showed that excessive polypharmacy (OR 3.63, 95% CI:1.51–8.74), a lower MMSE score (OR 1.29, 95% CI:1.12–1.48) and a higher GDS-15 score (OR 1.32, 95% CI:1.07–1.63) appeared to be independently connected to malnutrition or a risk of malnutrition. Conclusions: Malnutrition or a risk of malnutrition is a common problem among home care clients. Excessive polypharmacy, cognitive impairment and depressive symptoms increase malnutrition or the risk of malnutrition. To prevent a further decline in their health status, home care clients should be screened for malnutrition and the risk of malnutrition.
Key words: Malnutrition, home care, MNA, aged.
Introduction
Malnutrition is a common problem among older people in different healthcare settings (1-7). Malnutrition and unintentional weight loss are risk factors for mortality and have a negative influence on functional status, psychosocial well-being and quality of life among older people (1, 2, 4, 5). Malnutrition may lead to longer hospital stays and higher healthcare costs (2,8). Good nutritional status helps maintain functional abilities, allows independent living at home and helps avoid institutional care.
There are only a few studies on nutritional status and factors associated with malnutrition among home care clients (9-13). These studies have suggested that eating problems can lead to nutritional risk (9, 11). In Finland, the majority of home care is run by municipalities especially for persons with poor health and functioning. In addition older persons can buy home care services from the private firms (14). Home care consists of home helping (e.g. hygiene, cleaning, dressing) and home nursing (e.g. administration of drugs, wound care). Home care clients or their family caregivers prepare the food, if that is not possible, they can receive meals-on-wheels service. As far as we know, there are no studies concerning vulnerable home care clients that examine the relationship between nutrition, oral health and drug use. The aims of this study were to describe nutritional status and to identify factors associated with malnutrition and risk of malnutrition in a population-based sample of home care clients aged 75 years or older.
Methods
Study sample
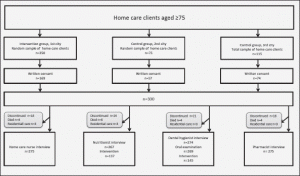
This cross-sectional study analysed baseline data collected as part of the Nutrition, Oral health and Medication (NutOrMed) study which seeks to evaluate the effects of six months of nutrition and oral health intervention on nutritional status, oral health, functional ability and hospitalisation use and costs among home care clients. Randomization among home care clients was done using the coded lists of home care clients and SPSS random sample tool. After that home care nurses asked if their clients wanted to participate. If the person had cognitive decline (Mini Mental State Examination (MMSE) <20) or poor capability of judgment, the information about the study was given to proxy who made the decision of participation. Of all randomized persons (n=440) 300 clients or their proxies gave a written consent.
Assessment
At the baseline, a nutritionist interviewed 267 participants, and 275 participants were interviewed by a dental hygienist, a home care nurse interview and a pharmacist. Figure 1 shows a flow chart of the study. If the participant had a cognitive impairment, the data were supplemented by a care giver. The nutritional screening was performed using the Mini Nutritional Assessment (MNA). The MNA is a validated and standardised screening tool for estimating the nutritional status of older people (15, 16). A total score >23.5 indicates normal nutritional status, scores of 17.0−23.5, a risk of malnutrition and <17.0, malnutrition (15,16). The participants were asked, “Do you have a feeling of dry mouth?” The question had three categories from no to continuously. Categories 1 (no) and 2–3 (occasionally and continuously) were combined for the analyses. Chewing problems were assessed by asking the participants, “Do you have chewing problems?” The question had two categories: “yes” and “no”. A pharmacist interviewed the participants at the baseline and recorded each prescription and over-the-counter drug used regularly and as needed on the basis of the interview, medication lists and medication packages. Drug use was categorised into two classes in univariate and multivariate analyses: 0−9 and 10 or more drugs (10 or more drugs indicate excessive polypharmacy). Biochemical measurements were plasma albumin, haemoglobin and vitamin B12. All loboratory tests were measured according to the standard protocols at the regional laboratory ISLAB (The Laboratory Centre of East of Finland). The information about the technique of sample collection, storage and transfer were provided in the Manual of ISLAB (17).
Depressive symptoms were evaluated on the 15-item geriatric depression scale (GDS-15), which is a validated tool (18). In this study, GDS-15 scores were categorised into two classes: scores of 0−4 and 5−15. Comorbidity was defined using a modified version of the Functional Comorbidity Index (FCI) (19,20). Data on the following 13 medical conditions were available: rheumatoid arthritis and other inflammatory connective tissue diseases; osteoporosis; chronic asthma or chronic obstructive pulmonary disease (COPD); coronary artery disease; myocardial infarction; heart failure; Parkinson’s disease; stroke; diabetes; depressive disorder; visual impairment; hearing impairment and dementing disease such as Alzheimer’s disease, vascular dementia, Lewy body dementia or other dementia. Each condition was given one point, and a higher FCI sum score represents greater comorbidity.
Performance in activities of daily living (ADL) was assessed with the 10-item Barthel Index (21) and in instrumental activity (IADL) (22), with the 8-item Lawton and Brody scale. The scoring for ADL is 0−100 and for IADL, 0−8, with higher scores indicating better functioning. Cognitive status was evaluated by using the Mini Mental State Examination (MMSE). The MMSE is a validity and reliability tool (23). The maximum score is 30, and scores < 24 indicate a cognitive disorder. Self-reported ability to walk 400 m was assessed by asking the participants, “Can you walk at least 400 meters?” The question had four response categories from dependent (0) to totally independent (3). Categories 0−1 (unable to walk or unable to walk independently) and 2−3 (able to walk independently with or without difficulties) were combined for the analyses.
Statistical analysis
The participants were categorised into two groups according to their MNA sum scores, using 24 points as the cut-off. Statistical comparisons between the groups were made using a chi-square or t-test, with a p-value of 0.05 considered significant. The results were significant if the p-value was < 0.05. The results were reported as means with standard deviations (SD) or frequencies with percentages. Univariate and multivariate (forward Wald selection) regression analyses were performed to identify demographical, clinical and functional factors associated with malnutrition or a risk of malnutrition (MNA ≤ 23.5). The data were analysed using SPSS 21.0 software.
Ethical approval
All the participants or their proxies gave written informed consent to participate in the study. The study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District, Kuopio, Finland.
Results
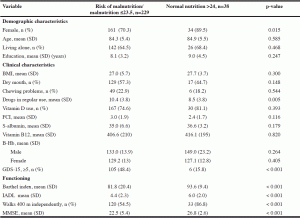
The mean age of the 267 homecare participants was 84.4 (SD 5.4) years and 73% (n=161) were female. A total of 229 (85.8%) participants had 23.5 points or less in the MNA screening (Table 1). The participants at risk of malnutrition or who were malnourished used more drugs and had a higher depressive score and lower Barthel Index, IADL and MMSE scores than the well-nourished participants.
SD = Standard deviation; BMI = Body Mass Index; GDS-15 = Geriatric Depression Scale; IADL = Instrumental Activities Of Daily Living; m = meter; MMSE = Mini Mental State Examination
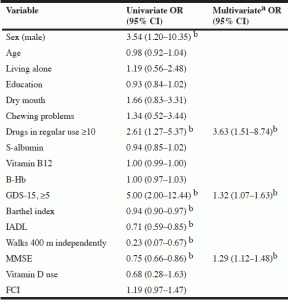
Table 2 Univariate and multivariate associations between patient characteristics and MNA scores of 23.5 and below
a. Forward wald selection. Only variables that entered the model are shown; b. statistically significant (p<0.05)
In the univariate analysis, excessive polypharmacy, a high GDS-15 score and low Barthel Index, IADL and MMSE scores and inability to walk 400 m independently were associated with a risk of malnutrition or with being malnourished. In the multivariate analysis, excessive polypharmacy (OR 3.63, 95% CI:1.51–8.74), a lower MMSE score (OR 1.29, 95% CI:1.12–1.48) and a higher GDS-15 score (OR 1.32, 95% CI:1.07–1.63) appeared to be independently connected to malnutrition or a risk of malnutrition.
Discussion
The prevalence of a malnutrition risk or malnutrition was 86%, which is higher than in previous studies of home care clients (9, 12). In an earlier Finnish cross-sectional study among home care clients, the prevalence of malnutrition risk or malnutrition was much lower, 51% (12). Reasons for this discrepancy may be that persons with a severe cognitive impairment were excluded, whereas we accepted all participants with a mild to severe cognitive impairment in our study. The prevalence of malnutrition risk or malnutrition varied in the other studies between 49%–69% (9, 10, 13). The differences in the prevalence of malnutrition risk or malnutrition may be due to differences in the demographic, socio-economic or inclusion criteria used in the present study and previous studies.
Polypharmacy was associated with malnutrition or a risk of malnutrition. The impact of polypharmacy on nutritional status is complex, and the two are interconnected (24). Polypharmacy is associated with a decline in nutritional status among community-dwelling older people (25, 26), but then malnutrition may worsen health and further increase drug use. Many drugs have adverse effects, such as changes in taste, loss of appetite or nausea, which can reduce food intake and thus lead to malnutrition (24, 27).
A decline in cognitive functioning was also associated with malnutrition or a risk of malnutrition. One previous study with home care clients confirms this association (11). Malnutrition is associated with cognitive impairment among older people living at home or in a nursing home (1, 28,2 9). However, the interrelationship between cognitive impairment and malnutrition or a risk of malnutrition is complex and reciprocal (29). Cognitive impairment causes inability to do shopping and prepare meals, and in more severe stage person can even forget to eat. Oral health problems also lead to malnutrition in persons with cognitive impairment (11, 30, 31).
There was a significant association between depressive symptoms and being at risk of malnutrition or being malnourished. Two previous study with home care clients have also found this association (9, 10). Lack of appetite, loss of interest in self-care, apathy and physical weakness can explain the association between malnutrition and depression (5). Depression can reduce food intake, and several studies suggest that this is a common cause of malnutrition in older people (9, 10, 32).
The strengths of the present study were its population-based design, validated instruments and multidisciplinary approach. The use of a random population-based sample from three municipalities meant it was likely to be representative of the target population. Finland is ethnically homogenous, and home care provided by municipalities is organised according to the national framework. The data on MNA were collected by a nutritionist, which improves internal reliability. In addition, the present study had no exclusion criteria regarding age and morbidity or the cognitive status of the home care clients. A limitation of this study is that the data were partly collected by several nurses, which could impact internal reliability. However, all the nurses were trained by the same person. This study was cross-sectional, so the relationships between risk factors and malnutrition status should not be considered causal.
Conclusions
Malnutrition or a risk of malnutrition is a common problem in home care clients. Risk was associated with polypharmacy, cognitive decline and depressive symptoms. To prevent a further decline in their health status, home care clients should be screened for malnutrition and the risk of malnutrition.
Acknowledgments: We would like to acknowledge the staff included in this study for their positive attitude during the data collection. IN received funding from North Savo Regional Fund.
Conflict of interest: No conflicts of interest.
Ethical Standards: The study protocol was approved by the Research Ethics Committee of the Northern Savo Hospital District, Kuopio, Finland. Informed consent was acquired from all the participants or their proxies.
References
1. Suominen M, Muurinen S, Routasalo P, Soini H, Suur-Uski I, Peiponen A, Finne-Soveri H, Pitkälä KH. Malnutrition and associated factors among aged residents in all nursing homes in Helsinki. Eur J Clin Nutr. 2005;59:578-83.
2. Verbrugghe M, Beeckman D, Van Hecke A, Vanderwee K, Van Herck K, clays E, Bocquaert I, Geurden B, Verhaeghe S. Malnutrition and associated factors in nursing home residents: A cross-sectional, multi-centre study. Clin Nutr. 2013;32:438-43.
3. Törmä J, Winblad U, Cederholm T, Saletti A. Does undernutrition still prevail among nursing home residents? Clin Nutr. 2013;32:562-8.
4. Ülger Z, Halil M, Kalan I, Yavuz BB, Cankurtaran M, Güngör E, Arioğul S. Comprehensive assessment of malnutrition risk and related factors in a large group of community-dwelling older adults. Clin Nutr. 2010;29:507-11.
5. Nykänen I, Lönnroos E, Kautiainen H, Sulkava R, Hartikainen S. Nutritional screening in a population-based cohort of community-dwelling older people. Eur J Public Health. 2013;23:405-9.
6. Kaiser MJ, Bauer JM, Rämsch C, Uter W, Guigoz Y, Cederholm T, Thomas DR, Anthony PS, Charlton KE, Maggio M, Tsai AC, Vellas B, Sieber CC; Mini Nutritional Assessment International Group. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58:1734-8.
7. Gordon AL, Franklin M, Bradshaw L, Logan P, Elliott R, Gladman JR. Health status of UK care home residents: a cohort study. Age Ageing. 2014;43:97-103.
8. Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235-9.
9. Saletti A, Johansson L, Yifter-Lindgren E, Wissing U, Osterberg K, Cederholm T. Nutritional status and a 3-year follow-up in elderly receiving support at home. Gerontology. 2005;51:192-8.
10. Kiesswetter E, Pohlhausen S, Uhlig K, Diekmann R, Lesser S, Heseker H, Stehle P, Sieber CC, Volkert D. Malnutrition is related to functional impairment in older adults receiving home care. J Nutr Health Aging. 2013;17:345-50.
11. Furuta M, Komiya-Nonaka M, Akifusa S, Shimazaki Y, Adachi M, Kinoshita T, Kikutani T, Yamashita Y.Interrelationship of oral health status, swallowing function, nutritional status, and cognitive ability with activities of daily living in Japanese elderly people receiving home care services due to physical disabilities. Community Dent Oral Epidemiol. 2013;41:173-81.
12. Soini H, Routasalo P, Lagström H. Characteristics of the Mini-Nutritional Assessment in elderly home-care patients.Eur J Clin Nutr. 2004;58:64-70.
13. Yang Y, Brown CJ, Burgio KL, Kilgore ML, Ritchie CS, Roth DL, West DS, Locher JL. Undernutrition at baseline and health services utilization and mortality over a 1-year period in older adults receiving Medicare home health services. J Am Med Dir Assoc. 2011;12:287-94.
14. National Institute for Health and Welfare (2010) Census of Home Services 30.11.2010. Available at: http://www.stakes.fi/ (accessed 14 December 2012).
15. Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: The mini nutritional assessment as part of the geriatric evaluation. Nutr Rev. 1996;54:S59-65.
16. Guigoz Y, Lauque S, Vellas BJ. Identifying the elderly at risk for malnutrition. The mini nutritional assessment. Clin Geriatr Med. 2002;18:737-57.
17. Patient Guide. The web manual of Eastern Finland Laboratory Centre (In Finnish). Available at: http://www.islab.fi/ (accessed 14 November 2014).
18. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. Clin Gerontolog. 1986;5:165-73.
19. Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595-602.
20. Tikkanen P, Nykänen I, Lönnroos E, Sipilä S, Sulkava R, Hartikainen S. Physical Activity at Age of 20–64 Years and Mobility and Muscle Strength in Old Age: A Community-Based Study. J Gerontol A Biol Sci Med Sci. 2012;67:905-10.
21. Mahoney FI, Barthel DW. Functional evaluation: The Barthel index. Maryland State Med J. 1965;14:61-5.
22. Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179-86.
23. Folstein M, Folstein S, McHugh P. Mini-mental state: a practical method of grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-98.
24. Zadak Z1, Hyspler R, Ticha A, Vlcek J. Polypharmacy and malnutrition. Curr Opin Clin Nutr Metab Care. 2013;16:50-5.
25. Jyrkkä J, Enlund H, Lavikainen P, Sulkava R, Hartikainen S. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf. 2011;20:514-22.
26. Heuberger RA, Caudell K. Polypharmacy and nutritional status in older adults: a cross-sectional study. Drugs Aging. 2011;28:315-23.
27. Pickering G. Frail elderly, nutritional status and drugs. Arch Gerontol Geriatr. 2004;38:174-80.
28. Lee KS, Cheong HK, Kim EA, Kim KR, Oh BH, Hong CH. Nutritional risk and cognitive impairment in the elderly. Arch Gerontol Geriatr. 2009;48:95-9.
29. Saka B, Kaya O, Ozturk GB, Erten N, Karan MA. Malnutrition in the elderly and its relationship with other geriatric syndromes. Clin Nutr. 2010;29:745-8.
30. Roqué M, Salvà A, Vellas B. Malnutrition in community-dwelling adults with dementia (NutriAlz Trial). J Nutr Health Aging. 2013;17:295-9.
31. Millán-Calenti JC, Tubío J, Pita-Fernández S, Rochette S, Lorenzo T, Maseda A. Cognitive impairment as predictor of functional dependence in an elderly sample. Arch Gerontol Geriatr. 2012;54:197-201.
32. van Bokhorst-de van der Schueren MA, Lonterman-Monasch S, de Vries OJ, Danner SA, Kramer MH, Muller M. Prevalence and determinants for malnutrition in geriatric outpatients. Clin Nutr. 2013;32:1007-11.