J. Lamure1,2, P. Brocker3, S.M. Schneider4, R. Collomp5, F. Bertin-Hugault6, P. Denormandie7, I. Prêcheur1,2,3
1. Department of Dentistry, Nice University Hospital, Nice, France; 2. Micoralis Laboratory, Faculty of Dentistry, University of Nice Sophia Antipolis, Nice, France; 3. Department of Geriatrics, Nice University Hospital, Nice, France; 4. Department of Nutrition, Nice University Hospital, Nice, France; 5. Pharmacy, Nice University Hospital, Nice, France; 6. Department of Geriatrics, Assistance Publique Hôpitaux de Lyon, Lyon, France; 7. Institut du bien vieillir Korian, Groupe Korian, Paris, France. Corresponding author: J. Lamure, Micoralis Laboratory, Faculty of Dentistry, 24 avenue des Diables Bleus, 06357 Nice cedex 4, France. Tel.: +33 4 92 00 11 00. E-mail address: drjulielamure@gmail.com
Abstract
Background & Aims: Many frail elderly patients are polymedicated. Whether they suffer from dysphagia (due to stroke, Parkinson’s disease, etc.) or cognitive troubles (due to Alzheimer’s disease, etc.), they are often given blended food, with drugs crushed and mixed into the food. Health Authorities recommend to crush and to administrate crushed drugs separately, for pharmacologic reasons, but the drugs are usually mixed together to facilitate ease of case by nursing staff. Crushed drugs can have a bad taste, leading to drug / food refusal, worsening malnutrition, but this qualitative aspect has been scarcely studied in geriatric populations. The present study aimed to evaluate the taste of the ten drugs most frequently prescribed in nursing homes, in order to determine which drugs are acceptable or not when crushed and mixed into food. Methods: This one-step observational study was designed like a food or wine tasting. A jury of healthy volunteers was recruited among medical staff (8 volunteers) and other people involved in food and gastronomy (8 volunteers, including a starred Chef). Every tablet or capsule was mixed into 100 mL of berry-flavored jelly or apple sauce. It was a blind tasting of 24 verrines, containing the ten drugs randomly distributed, a control without drug and a combination of the 6 top-list drugs. Twelve jelly verrines were followed by 12 apple sauce verrines. Tasters spat the spoonful content out after they had assessed its taste. Each verrine was scored from 0 (bad taste) to 10 (good). Qualitative and free comments were also recorded. Results: The lowest scores were attributed to the combination of paracetamol, alprazolam, furosemide, levothyroxine sodium, memantine and zopiclone (1.5 + 1.6; 0 to 5), followed by zopiclone (1.9 + 2.3; 0 to 8), clopidogrel (4.3 + 2.1; 1 to 7) and paracetamol (4.6 + 1.8; 1 to 8). All these drugs had a long-lasting bitterness. Zopiclone mixed and alone was qualified as unbearable and one participant exhibited nausea by taking it. Five participants did not take lunch after the study for lack of hunger (5/16: 31.3 %). Drug-free jelly and apple sauce were scored 6.7 + 1.4 (4 to 9) and 7.1 + 1.1 (5-9.5), respectively. Other scores ranged from 6.1 to 7.9, for alprazolam, ramipril, oxazepam, levothyroxine sodium, donezepil and furosemide. Conclusions: The taste of some drugs may be unbearable when they are crushed and mixed into food, and caregivers should avoid mixing a bad-tasting drug with the other drugs. There are wide differences of taste acceptability from one person to another. Thus, during workshops, every patient could taste once separately any single drug in his prescription list. If a bad taste leads to drug refusal, caregivers should inform physicians and pharmacists, who in turn should seek alternative medical solutions (drug discontinuation or substitution). Caregivers could also seek alternative food or administration conditions. On a mid-term basis, pharmaceutical companies should also develop specific pharmaceutical forms, as they do for children.
Key words: Food-drug interactions, frail elderly, malnutrition, swallowing disorders, taste.
Introduction
Elderly people frequently suffer from chronic diseases and are consequently often polymedicated. In nursing homes and geriatric hospital wards, they are administered a daily average of 6 to 8 drugs, corresponding to 6 to 20 tablets, pills or capsules (1). The prevalence of dysphagia increases with age: at least 15% of the elderly population and over 50% of residents in nursing homes are affected by dysphagia due to stroke, cancer, Parkinson’s disease, Alzheimer’s disease, Sjögren’s syndrome and some medications that can cause xerostomia. Dysphagia increases the risk of aspiration pneumonia. The consequent beverage and food refusal can lead to dehydration, anorexia, malnutrition and potentially even death. As a precaution, these patients are often given blended food (2). Nursing staff is also obliged to crush tablets, to open capsules, and to mix drugs into textured food, frequently made with blenders.
Crushing drugs can induce chemical (e.g. oxidization, acid-basic interaction) and pharmacologic problems (such as with gastro-resistant tablets) (3). Listing of drugs authorized for crushing and consensual recommendations for their administration have been published by several groups of experts (4, 5). According to recommendations: (1) physicians should limit drug prescription, (2) pharmacists should propose alternative formulation such as oral drops whenever possible, and (3) nurses should only crush authorized drugs, they should do so separately just before administration and they should mix them into separated food servings (3). Usually, nurse’s aides are charged with giving both food and drugs to patients, and so this task often falls on them. Soft/liquid and sweet food under a small volume is generally preferred, such as jelly, apple sauce or dairy products (100 to 125 mL/serving).
In nature, many poisons are alkaloids stimulating bitter taste receptors, and bitterness mediates an aversive response to toxic food (6). Many drugs have a bitter taste, too, and when they are crushed into food, there is a risk of developing food aversion. But the sensorial aspect of crushing drugs has scarcely been studied in geriatric populations. Published studies focus mainly on anti-inflammatory medicines (prednisolone, diclofenac), antimalarial medicines (mefloquine, artemether, lumefantrine) and antihypertensives (amlodipine, candesartan, etc.), generally involving pediatric patients (5–12)
The present study aimed to evaluate the taste of the ten drugs most frequently prescribed in nursing homes, in order to determine which drugs are acceptable or not when crushed into food, from a sensorial point of view. The medical objective is to limit drug refusal and anorexia. The study was designed like any food or wine tasting, with a jury of healthy volunteers recruited among medical staff (eight volunteers) and other people involved in food and gastronomy field (eight volunteers).
Material and methods
The study was a one-step observational study carried out in June 2014 in the Department of Clinical Research of the Nice University Hospital. It was a phase I study with a cohort of 16 volunteers (Table 1). Investigators and participants were unpaid. Participants were recruited by the investigators in professional and personal settings. Non-inclusion criteria were untreated severe disease, allergy to any of the drugs to be tested, age over 70, pregnancy or breast-feeding. Current drug prescription was not an exclusion criterion. The morning of the study, just before the test session, the main investigator in charge of the study performed medical consultations and obtained written informed consent from all of the participants. They were given an emergency hospital phone number available night and day for a one-week follow-up, if necessary.
The ten drugs tested were selected as the top-list of the drugs prescribed in 2013 in the 596 nursing homes of the Groupe Korian, in France, Germany, Italy and Belgium. Only tablets or capsules with crushing authorization were selected (4, 5) . Whenever applicable, the lowest dosage was selected. The drugs were provided by the hospital pharmacy. Every tablet or capsule was mixed into 100 mL of berry-flavored jelly (Valade, France) or apple sauce (Andros, France). A volume of 5 mL of each preparation was distributed in small transparent plastic cocktail cups (verrines) and served with 1 mL-containing disposable coffee spoons. We assumed that each volunteer would taste two spoons of each mixture, corresponding to 1/50 of every tablet or capsule. After mouth rinse with flat bottled water (Evian, France), the residual quantity of food available for swallowing was estimated to 0.1 mL. The residual quantity of drugs available for swallowing was thus evaluated to 1/500 of every drug/verrine tasted. There was a blind tasting of 24 verrines, containing ten drugs, a control without drug and a combination of the 6 top-list drugs, corresponding to 12 jelly verrines followed by 12 apple sauce verrines. After tasting, tasters spit out the spoonful contents into a disposable plastic cup. The protocol was approved by the local Ethics Committee and registered by Health Authorities under Eudract n° 2013-003461-34.
This pilot study involved mostly non-professional food tasters. The investigation was limited to 16 participants, as it was a descriptive study without group comparison and no minimal number was required for statistical analysis. The main outcome assessment was a score attributed to each verrine containing crushed drugs or negative controls, ranging from 0 (bad taste) to 10 (good taste). The results were expressed as a mean score and standard deviation calculated for the 16 volunteers. Secondary outcomes were a tentative attribution to common flavors (sugary, sweet, sour, bitter, salty, astringent, prickling, aromatic, etc.) and free comments (6). All participants were recalled for possible post-study comments.
The order of drug serving was randomized in two blocks (jelly and apple sauce). Each verrine was assigned a number ranging from 1 to 24, beginning with jelly (1-12) and ending with apple sauce (13-24). The drugs were crushed and mixed by a nurse of the Clinical Research Department in a separate room. Participants were blinded to verrines contents and were not allowed to voice their tasting evaluation aloud. Each mouthful was spat into a disposable opaque plastic cup. In addition to bottled water, participants were offered white bread and green apples to clean their mouth between verrines, as with wine and food tasting protocols.
Results
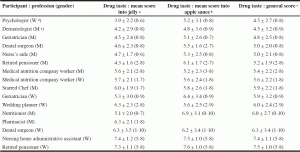
Sixteen participants eligible for the study were recruited from Nice (France) and Monaco (Principality of Monaco). The cohort was comprised of four physicians (two geriatricians, a nutritionist and a dermatologist), a pharmacist, two dental surgeons, a nurse’s aide, a psychologist, a Michelin starred Chef, a wedding planner, a nursing home administrative assistant, two retired pensioners and two members of a company specializing in oral nutritional supplements. The pharmacist had to leave the protocol following the first half of the study (immediately following the jelly test portion) because of professional reasons. There were nine men and seven women, aged 27 to 69. As for a formal tasting, participants were asked to avoid morning coffee or tea as well as perfumed cosmetics before the test. In order to preserve their anonymity, they were not introduced to each other. The drug tasting lasted from 9 to 10:30 in the morning. The mean scores attributed by the 16 volunteers are detailed in Table 1.
a,b,c Scoring ranged from 0 (bad taste) to 10 (good taste): mean score, standard deviation and extreme values attributed to the 12 verrines containing jelly a, to the 12 verrines containing apple sauce b and to the 24 verrines (jelly and apple sauce) c ; d W: women; e M: men
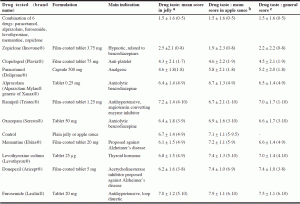
The randomized order of tasting in jelly was as follows: ramipril, alprazolam, combination of six drugs, zopiclone, paracetamol, furosemide, levothyroxine sodium, memantine, oxazepam, clopidogrel, negative control (jelly) and donezepil. There was a 5 min pause between the tasting of jelly and apple sauce verrines. The randomized order of tasting in apple sauce was as follows: furosemide, clopidogrel, donezepil, memantine, negative control (apple sauce), paracetamol, oxazepam, levothyroxine sodium, alprazolam, combination of six drugs, zopiclone and ramipril. The lowest scores were attributed to the combination of six drugs, followed by zopiclone, clopidogrel and paracetamol. The scores attributed to the ten drugs are detailed in Table 2.
a,b,c Scoring ranged from 0 (bad taste) to 10 (good taste): mean score, standard deviation and extreme values attributed to the 12 verrines containing jelly a, to the 12 verrines containing apple sauce b and to the 24 verrines (jelly and apple sauce)
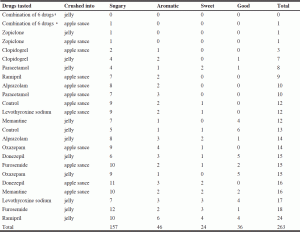
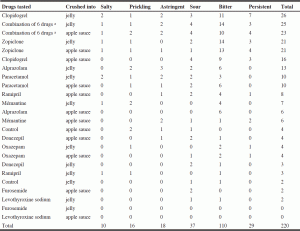
Qualitative evaluation is detailed in Table 3 and 4. Table 3 and 4 record how many times each qualifier was mentioned on the record cards, either as a positive (pleasant) or a negative (unpleasant) comment. In addition to the score, some drugs were attributed qualitative comments. Quantitative (Table 2) and qualitative (Table 3 and 4) evaluations allowed similar ranking of the drugs, as regards the pleasure (or lack thereof) to taste them. The psychologist recorded that she almost experienced nausea by taking the verrines containing zopiclone alone in jelly as well as combined with other medications in jelly, but not into apple sauce. Nearly one third of participants (5/16 or 31.3%) did not wish to take lunch after the study because of anorexia. There were no other side effects reported during the week following the study. The words «unedible, unbearable, unacceptable, very bad, very unpleasant, terrible, not bearable, impossible to ingest, no way» were used to describe the bitterness and long-lasting bitter taste of zopiclone, alone and combined with other drugs. Clopidogrel and paracetamol also had negative comments, but not as severe.
a. Combination of six drugs: paracetamol, alprazolam, furosemide, levothyroxine sodium, memantine, zopiclone
a. Combination of six drugs: paracetamol, alprazolam, furosemide, levothyroxine sodium, memantine, zopiclone
Discussion
The key result of this study was the wide difference of taste among the ten crushed drugs tested. One crushed drug had an unbearably strong and long-lasting bitterness (zopiclone), and two others had a very pronounced bitterness (clopidogrel, paracetamol). A combination of six drugs containing both zopiclone and paracetamol elicited the worst response. Conversely, the seven other drugs tested were scored from acceptable to good (alprazolam, ramipril, oxazepam, memantine, levothyroxine sodium, donezepil and furosemide). Short or long-lasting bitterness were the main concerns, but unpleasant tastes such as salty, prickling, astringent and sour were also reported. There was a huge variability between drugs, but also, though in a lesser way, between users. The qualitative evaluation, detailed in Table 3 and 4, is not really relevant in this study. However the qualitative description of the taste is part of regular tasting session protocols and had to be done in this study in order to be complete. We won’t do further investigations on qualitative descriptions.
Being a pilot study, this work suffered from several biases and inaccuracies. Among these was the limited number of participants; the bitter drugs probably altered the taste perception of the subsequent verrines; a series of 24 consecutive tests was probably excessive; the crushed drugs were mixed only into jelly and apple sauce but not in dairy products; a 5-point scale or less could have been more reliable; etc. (7–10). In 2014, Uestuener et al. (10) designed a protocol to taste acceptability of amlodipine and candersartan. According to these authors, there was no taste difference between pulverized brand-name and generics. The participants were health care professionals, including 19 nurses and 12 physicians (10). The originality of the present study was to involve people working in the field of food and gastronomy in a pharmacological study. All in all, this study was unpleasant but safe for participants and, in addition to zopiclone, clopidogrel and to a lesser extent paracetamol, other drugs with a bad taste could be identified. Last, age and disease are known to induce taste modifications (1–3), and therefore reports from healthy volunteers may not match results in the target population.
Despite recommendations, it is commonly observed that several drugs are crushed together and administered in the same food (3). Actually, a single drug with a bad taste can induce patient’s refusal, leading to non-compliance with the entire regimen, followed by meal refusal, anorexia and malnutrition. In turn, malnutrition increases the risk of infections, falls, bed sores and depression, the length of hospital stay and loss of autonomy and thus increases drugs consumption (11). Several approaches could be possible to combat against this situation.
The first approach would be to limit polymedication whenever possible. In an elderly population, the identification of drugs with a bad taste could also be a first-line measure. The use of taste sensing technology could aid in the design of new drug formulations with better tastes, but technology cannot replace individual evaluation (12). A questionnaire related to appetite, hunger and sensory perception might not be a reliable tool in a geriatric population, due to the high prevalence of cognitive impairment (13). Due to the huge variability that we observed between participants and between drugs, it appears necessary for dysphagic elderly person to sample each drug in his/her prescription list, in order to identify unacceptable drugs. In case of cognitive troubles, nurse’s aides are used to interpret patients’ body language for food refusal and this approach would likely be easier than to find alternative solutions for an entire prescription list. Drug tasting could be scheduled among other workshops, organized by family members, dieticians or psychologists, for instance.
Physicians and pharmacists are not always aware of the difficulties encountered by nurses, nurse’s aides or family members in the administration of medications. The present study may help promote communication, and caregivers should inform physicians and pharmacists of drug refusal. In some cases, blended food can be considered as a real mistreatment (14). In the present study, the free comments of several participants revealed that the addition of bad-tasting drugs into blended food could be considered as another mistreatment and should be avoided.
In case of drug refusal, physicians and pharmacists might provide a range of alternative solutions. Attention should be focused on the problematic drug, while other medications might be well tolerated. Medical solutions could be: (1) discontinuing the drug, (2) if they are available, the prescription of alternative molecules with a better taste, or (3) the prescription of other dosing formulations (pediatric formulation, powder, liquid, suppository, patch, sustained-release formulation, etc.) (3, 15–19).
Dietary supplements, such as apple sauce vs. jelly (Table 1-4) may help make molecules more palatable. Jam, yogurts or other dietary products may be valuable alternatives. Sugar seems consensual to mask bitterness [20], but a frequent limit is diabetes mellitus. There is also an increased risk of dental caries, particularly with patients who are given polypharmacy and who frequently suffer from drug-induced xerostomia. Zopiclone, for instance, is a hypnotic medicine given at bed-time after oral hygiene care, and evening sugar intake cannot be recommended to dentate patients in such conditions. Other sweeteners or suspending agents are also available to improve drug palatability (21). The nurse’s aide participating in the study made the recommendation to concentrate the crushed drugs into one or two spoons of jam, rather than to dilute them in a larger volume. Breakfast milk, porridge, soup, mashed vegetables and sweets should be avoided. The chef recommended chewing white bread or green apple after tasting, instead of drinking water to clean the mouth, because water can increase bitterness.
Finally, since the Prescription Drug User Fee Act and Food and Drug Administration Amendments Act of 2007, it is mandatory for pharmacological companies to test new medicines in clinical assays involving pediatric populations. Consequently, several companies proposed physical alteration to mask bitter taste and improve treatment compliance in children. Current solutions are: granule formulation (17), suppositories/mucoadhesive gels (15), micro- or nanostructures and nanohydrids (19, 22), hot melt extrusion (19), cyclodextrin inclusion alone or combined with lipid coating or ion exchange resin (23, 24). Similar to having these newly developed “children-friendly” drugs, it would be useful to have a strategy dedicated to the elderly population (7–9, 15–18, 25–27).
In conclusion, the taste of some drugs may be unbearable when they are crushed and mixed into food, and caregivers should avoid mixing bad-tasting drugs with other more palatable drugs. There are wide differences of taste acceptability from one person to another. Thus, during workshops, each patient could taste once separately any single drug in his or her prescription list. In case of drug refusal, caregivers should inform physicians and pharmacists, who in turn should seek medical alternatives (drug discontinuation or substitution). Caregivers could employ alternative food serving or administration conditions. Pharmaceutical companies should also develop specific medicines for the older populations, in parallel with “children-friendly” medicines.
Acknowledgements: The Authors thank Prof Philippe Bahadoran, the medical staff of the Center of Clinical Research (Nice University Hospital) and the 16 volunteers. They thank Dr Dale Rosenbach for manuscript revision (Clinical Instructor & Course Director, Periodontics & Implant Dentistry at Woodhull Medical Center, New York, USA).
Funding sources: Solidages (Nice, France), Institut du bien vieillir Korian (Paris, France) and Nice University Hospital (Nice, France). Sponsors played no role in the study.
Conflict of interest: The authors declare no conflict of interest with the present study.
Ethical Standards: The protocol was approved by the “CPP Sud Méditerranée V” Ethics Committee and registered by Health Authorities under Eudract n° 2013-003461-34.
References
1. Patterson SM, Hughes C, Kerse N, et al. (2012) Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 5:CD008165. doi: 10.1002/14651858.CD008165.pub2
2. Sura L, Madhavan A, Carnaby G, Crary MA (2012) Dysphagia in the elderly: management and nutritional considerations. Clin Interv Aging 7:287–298. doi: 10.2147/CIA.S23404
3. (2014) Crushing tablets or opening capsules: many uncertainties, some established dangers. Prescrire Int 23:209–211, 213–214.
4. Utilisation des antisécrétoires gastriques et autres médicaments antiulcéreux dans le Département de Gériatrie des Hôpitaux… – cappinfo26.pdf. http://pharmacie.hug-ge.ch/infomedic/cappinfo/cappinfo26.pdf. Accessed 11 Mar 2015
5. couper,ecraser, ouvrir_finale – Couper_ouvrir_ écraser_mars_2013.pdf. http://www.phel.ch/secure/Pdf/Couper_ouvrir_%20%C3%A9craser_mars_2013.pdf. Accessed 11 Mar 2015
6. Levit A, Nowak S, Peters M, et al. (2014) The bitter pill: clinical drugs that activate the human bitter taste receptor TAS2R14. FASEB J Off Publ Fed Am Soc Exp Biol 28:1181–1197. doi: 10.1096/fj.13-242594
7. Abdulla S, Sagara I (2009) Dispersible formulation of artemether/lumefantrine: specifically developed for infants and young children. Malar J 8 Suppl 1:S7. doi: 10.1186/1475-2875-8-S1-S7
8. Ferrarini A, Bianchetti AA, Fossali EF, et al. (2013) What can we do to make antihypertensive medications taste better for children? Int J Pharm 457:333–336. doi: 10.1016/j.ijpharm.2013.07.054
9. Ali AA, Charoo NA, Abdallah DB (2014) Pediatric drug development: formulation considerations. Drug Dev Ind Pharm 40:1283–1299. doi: 10.3109/03639045.2013.850713
10. Uestuener P, Ferrarini A, Santi M, et al. (2014) Taste acceptability of pulverized brand-name and generic drugs containing amlodipine or candesartan. Int J Pharm 468:196–198. doi: 10.1016/j.ijpharm.2014.04.035
11. Gastalver-Martín C, Alarcón-Payer C, León-Sanz M (2014) Individualized measurement of disease-related malnutrition’s costs. Clin Nutr Edinb Scotl. doi: 10.1016/j.clnu.2014.10.005
12. Ikezaki H (2014) [Development of taste sensor for bitterness evaluation of drugs]. Yakugaku Zasshi 134:313–316.
13. Savina C, Donini LM, Anzivino R, et al. (2003) Administering the “AHSP Questionnaire” (appetite, hunger, sensory perception) in a geriatric rehabilitation care. J Nutr Health Aging 7:385–389.
14. Pouyssegur V, Brocker P, Schneider SM, et al. (2015) An innovative solid oral nutritional supplement to fight weight loss and anorexia: open, randomised controlled trial of efficacy in institutionalised, malnourished older adults. Age Ageing 44:245–251. doi: 10.1093/ageing/afu150
15. Jannin V, Lemagnen G, Gueroult P, et al. (2014) Rectal route in the 21st Century to treat children. Adv Drug Deliv Rev 73:34–49. doi: 10.1016/j.addr.2014.05.012
16. Ivanovska V, Rademaker CMA, van Dijk L, Mantel-Teeuwisse AK (2014) Pediatric drug formulations: a review of challenges and progress. Pediatrics 134:361–372. doi: 10.1542/peds.2013-3225
17. Kibleur Y, Dobbelaere D, Barth M, et al. (2014) Results from a Nationwide Cohort Temporary Utilization Authorization (ATU) survey of patients in france treated with Pheburane(®) (Sodium Phenylbutyrate) taste-masked granules. Paediatr Drugs 16:407–415. doi: 10.1007/s40272-014-0081-5
18. Liu F, Ranmal S, Batchelor HK, et al. (2014) Patient-centred pharmaceutical design to improve acceptability of medicines: similarities and differences in paediatric and geriatric populations. Drugs 74:1871–1889. doi: 10.1007/s40265-014-0297-2
19. Kaushik D, Dureja H (2014) Recent patents and patented technology platforms for pharmaceutical taste masking. Recent Pat Drug Deliv Formul 8:37–45.
20. Mennella JA, Reed DR, Mathew PS, et al. (2015) “A spoonful of sugar helps the medicine go down”: bitter masking by sucrose among children and adults. Chem Senses 40:17–25. doi: 10.1093/chemse/bju053
21. Wilken MK, Satiroff BA (2012) Pilot study of “miracle fruit” to improve food palatability for patients receiving chemotherapy. Clin J Oncol Nurs 16:E173–177. doi: 10.1188/12.CJON.E173-E177
22. Coupland JN, Hayes JE (2014) Physical approaches to masking bitter taste: lessons from food and pharmaceuticals. Pharm Res 31:2921–2939. doi: 10.1007/s11095-014-1480-6
23. Ge Z, Yang M, Wang Y, et al. (2014) Preparation and evaluation of orally disintegrating tablets of taste masked phencynonate HCl using ion-exchange resin. Drug Dev Ind Pharm. doi: 10.3109/03639045.2014.914529
24. Samprasit W, Akkaramongkolporn P, Ngawhirunpat T, et al. (2014) Formulation and evaluation of meloxicam oral disintegrating tablet with dissolution enhanced by combination of cyclodextrin and ion exchange resins. Drug Dev Ind Pharm 1–11. doi: 10.3109/03639045.2014.922573
25. Milani G, Ragazzi M, Simonetti GD, et al. (2010) Superior palatability of crushed lercanidipine compared with amlodipine among children. Br J Clin Pharmacol 69:204–206. doi: 10.1111/j.1365-2125.2009.03580.x
26. Lucas-Bouwman ME, Roorda RJ, Jansman FG, Brand PL (2001) Crushed prednisolone tablets or oral solution for acute asthma? Arch Dis Child 84:347–348.
27. Schlagenhauf P, Adamcova M, Regep L, et al. (2011) Use of mefloquine in children – a review of dosage, pharmacokinetics and tolerability data. Malar J 10:292. doi: 10.1186/1475-2875-10-292