A.J. Fortes Ferreira1,3, M. Eickemberg2,3,4, A.K. Carneiro Roriz3,5, J.M. Barreto Medeiros5, L. Barbosa Ramos5,3
1. School of Nutrition, Federal University of Bahia, Brazil; 2. Bahiana School of Medicine and Public Health, Brazil; 3. Study and Intervention Center on Aging Area, Brazil; 4. Institute of Public Health. Federal University of Bahia, Brazil; 5. Department of Nutritional Science. School of Nutrition. Federal University of Bahia, Brazil. Corresponding author: Andrêa Jacqueline Fortes Ferreira, Nutrition School, Federal University of Bahia, Avenue Araujo Pinho, No. 32, Canela, CEP 40110-150, Salvador, Bahia, Brazil. Phone: (+55) 71991392900. Email: andrea18f@gmail.com
Jour Nursing Home Res 2017;3:38-42
Published online March 17, 2017, http://dx.doi.org/10.14283/jnhrs.2017.6
Abstract
Objective: Evaluate the phase angle and the factors associated with their values in institutionalized Brazilians elderly. Design: cross-sectional study. Participants and Settings: Study with 213 subjects aged ≥ 60 years, of both sexes, residents in long-term care facilities for the elderly. Measurements: The phase angle was determined by examining the bioelectrical impedance. Body mass index was used to evaluate the anthropometric nutritional status and skeletal muscle mass index to estimate skeletal muscle mass reserve. To examine the factors related to the phase angle was used Poisson regression with robust variance. Results: More than half of the elderly (50.7 %) presented phase angle below normal values, with higher prevalence among females (60.9 %). Men showed median values of phase angle and phase angle percentiles superior to women. Low phase angle values were associated with sex (PR: 2.08; 95% CI: 1.06 to 4.07 ), longevity (PR: 1.92 ; 95% CI: 1.04 to 3.52 ) and sarcopenia severe (PR: 1.26 ; 95% CI: 1.05 to 1.50). Conclusion: The occurrence of PA below normal values was high, especially among women and was associated with sex, severe sarcopenia and longevity, demonstrating the possible role of PA as elderly identification tool with impaired skeletal muscle mass reserve.
Key words: Phase angle, elderly, geriatric long-term care facilities.
Introduction
In recent years, the phase angle (PA) has been proposed as a prognostic tool and survival in several clinical situations for reflecting changes in the electrical properties of tissues, as well as changes in the integrity and permeability of cell membranes. These changes can result in nutritional and functional impairment, affecting an individual’s health and it is marked for the natural aging process (1-2).
The age, sex and body mass are determining factors of the values of PA (1,3), and these values can be positively influenced by skeletal muscle reservation and regular practice of physical activity. In the elderly, the skeletal muscle mass reserve decreases, regular physical activity also tends to decrease and the disease burden increases, negatively influencing the phase angle values (4).
In the case of institutionalized elderly and their health and nutrition, possibly they have greater impairment of cellular integrity and hence major changes in the values of PA when compared to the elderly living in the community (4). On the other hand, it is not well defined in the literature the cutoff point between PA values considered physiological and pathological, making it difficult to interpret. However, studies are scarce on this theme, which makes difficult the understanding of the role of this biomarker in the health of older people as well as the monitoring of changes, resulting from the aging process. Thus, this study evaluated the PA and the factors associated with their values in institutionalized elderly Brazilians.
Methodology
Study design
The Cross-sectional study, included in a larger research project, called «Multidimensional evaluation of elderly residents in long term care facilities in the city of Salvador, Bhia», developed by the Center of study and intervention on Aging, of the Nutrition school of Federal University of Bahia.
Sample
The largest study sample occurred in three steps. In the first stage, we identified 29 long-term care facilities for the elderly, which were located in 10 districts of the 12 existing ones, in the urban area. In the second phase, the number of elderly that would be part of the study was determined by health district. The number of elderly was proportional to the total population of elderly residents in each health district, which secured a sample power of 80% and a significance level of 5%, totalizing 412 elderly of both sexes. In the third stage, long-term care facilities for the elderly and elderly were selected by simple random sampling.
Due to the protocol for the examination of bioelectrical impedance and the selected variables, the final sample consisted of 213 individuals.
Eligibility criteria
Participated in the study, elderly patients (≥ 60 years), of both sexes, residents in long term care facilities for the elderly in the city of Salvador and did not show any inability to perform the bioelectrical impedance, or use a pacemaker or cardiac defibrillator, amputation of members, presence of ascites, visible swelling and inability to weight measurement.
Data collect
Data were collected between the months of October 2012 and November 2013, by a team trained by standard techniques. The elderly or those responsible filled a previously standardized, pre-coded form, containing information relating to sociodemographic characteristics (gender, age, race/skin color and time of institutionalization) and clinical (arterial hypertension, type II diabetes mellitus and dyslipidemia). Other clinical variables of interest were collected by anthropometric assessment and examination of bioelectrical impedance. The protocol for performing bioelectrical impedance consisted: fasting for at least four hours prior emptying of the bladder at the time of testing, abstain from strenuous exercise eight hours before the test and alcohol intake in the last 48 hours. The examination was performed in fresh air, with the elderly lying down in nonconductive surface and free of metal, following the criteria proposed by Kyle et al. in 2004 (5).
The Research Ethics Committee of the Nutrition School of Federal University of Bahia, assent 11/12, approved the study. The results of evaluations were returned to institutions and when necessary the elderly were referred for treatment in outpatient nutrition and geriatrics of the Federal University of Bahia.
Variables
Outcome variable: phase angle
The parameters for calculating the PA (resistance and reactance) were obtained by examining the bioelectrical impedance, brand Biodynamic model 450. For the calculation of the PA values, we used the formula proposed by Baumgartner, Chumlea and Roche (6), where:
PA (in degrees) = arc tangent reactance / resistance x 180 / π. Values less than five degrees were considered below normal (3-4).
Covariates
The covariates were age, sex, race/skin color, time of institutionalization, body mass index and skeletal muscle mass index.
The elderly were grouped into three age groups: 60-69 years, 70-79 years and above 80 years old. The race/skin color was self-reported by the elderly when completing the questionnaire, according to the classification of the Brazilian Institute of Geography and Statistics, in white, yellow, brown, black and indigenous (7). For the analysis, the variable was grouped into two categories: white and non-white. The time of institutionalization of the elderly were categorized into three groups: less than or equal to one year, between one to five years and over five years.
To evaluate the anthropometric nutritional status of the elderly used the body mass index (BMI), according to the formula proposed by the World Health Organization (WHO) in 1995 (8). The weight and height were obtained according to the techniques recommended by Jellife (9) and Chumlea, Roche and Steinbaugh (10), respectively. The body mass index of the subjects was classified according to the cutoff points proposed by the Pan American Health Organization (11). For data analysis, we used two categories: low weight (BMI <23 kg / m²) and without low weight (BMI ≥ 23 kg / m²).
The skeletal body mass index (SMI) was obtained through standardization of skeletal muscle mass by height, according to the formula proposed by Lauretani et al. (12): SMI (kg / m²) = skeletal muscle mass (kg) / height² (m²).
For determination of skeletal muscle mass (SMM) was used the equations proposed by Janssen et al. (13), adjusted for sex (0 – female, 1 – male) and age (years): SMM (kg) = [(Hight² (cm) / R (ohm) x 0401) + (Sex x 3.825) + (Age x – 0071)] + 5.102. The values of skeletal muscle mass index were stratified according to the cutoff points established by Janssen et al. (14): severe sarcopenia ≤8.5 kg/m², moderate sarcopenia 8.51-10.75 kg/ m², normal muscle ≥ 10.76 kg/ m² for men; and severe sarcopenia ≤ 5.75 kg/m², moderate sarcopenia 5.76-6.75 kg/m², normal muscle ≥ 6.76 kg/m² for women.
The variables arterial hypertension, diabetes mellitus type II and dyslipidemia were self-reported by the elderly and only used to characterize the population. In addition, these variables were not part of the data modeling, since the analyses of these possible associations was not the aim of this study.
Statistical analysis
We used the Kolmogorov-Sminorv test to analyze the normality of data and graphical analysis of histograms and box-plots. Through the description of the median, interquartile range and values of percentiles of covariates, the sample was characterized according to sex and age group. The prevalence of clinical variables arterial hypertension, diabetes mellitus type II and dyslipidemia have been described. The difference in medians of continuous variables (age, body mass index, skeletal muscle mass index, time of institutionalization and PA) between the sexes was analyzed using the Mann-Whitney test. The association between race / skin color and the outcome variable was verified by Pearson Chi Square test.
To investigate the relationship between the PA and the covariates (gender, age, race/skin color, body mass index, skeletal muscle mass index and time of institutionalization), we used the Poisson regression model with robust variance, estimating the ratio of gross and adjusted prevalence and their respective confidence intervals at 95%. We opted for the modeling that considers the possible cluster effect relating to the aggregation of individuals in long-term care facilities for the elderly. Regression models were constructed by the procedure of elimination (backward), which is part of a full model of equation.
A 5% significance level for all analyzes was adopted. We used the statistical software STATA, version 12.
Results
Most patients were female (73.24%) and non-white race/skin color (70%). According to the body mass index, 54% (n = 115) of the elderly presented low weight, 63% (n = 36) of men and 50.6% (n = 79) of women. Regarding the profile of chronic diseases, 58.3% (n = 119) of subjects had arterial hypertension,
21.7% (n = 43) diabetes mellitus type II and 14.8% (n = 29) dyslipidemia.
Men showed mean values of PA and skeletal muscle mass index higher than those found among the women, with a statistically significant difference (p <0.0001) (Table 1).
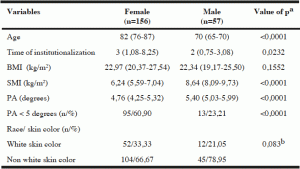
Table 1
Characteristics of institutionalized elderly, according to sex, Salvador, Bahia, Brazil, 2012-2013
Note: BMI – Body Mass Index; SMI – Skeletal Muscle Mass Index, PA – Phase Angle; Age, time of institutionalization, BMI and SMI are expressed as median (interquartile range). a – Mann -Whitney test; b – Test Chi square Pearson.
The overall prevalence of PA below normal values in the evaluated elderly was 50.70% (n = 108). Analyzing the values of PA according to race/skin color, it was observed that 57.81% (n = 37) of individuals of white race/skin color had PA values below normal, while 47.65 % (n = 71) of subjects of nonwhite race/skin color had PA values below the normal range, but the difference was not significant.
In the analysis of percentiles, Table 2 shows that the PA values tended to superiority for males compared to females in all quintiles. The PA percentile values tended to decrease as age increased in both sexes. It is observed that around 50 % of the women aged ≥ 80 years had PA values below normal ranges (5.0 degrees).
Gross associations between PA below normal values and the covariates of the study are shown in Table 3. PA values below normal significantly associated with female gender and age ≥ 80 years. Older females showed occurrence 2.67 (PR: 2.67; 95% CI: 1.20 to 5.90) times higher PA values below normal compared to men. Analyzing age, seniors over 80 years had occurred 161% (PR: 2.61; 95% CI: 1.43 to 4.74) higher PA below the normal range when compared to older people aged 60- 69 years. The other variables were not associated with PA outcome.
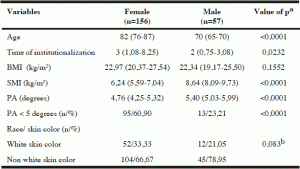
Table 2
Percentile of phase angle in institutionalized elderly, according to sex and age, Salvador , Bahia , Brazil , 2012-2013
In model one, which included all variables, sex and age group maintained the association with PA values below normal values, adjusted for other variables in the model. In the model two covariates sex and age still remained in statistically significant association with the outcome, PA below normal values, adjusted for covariates in the model, with a small increase in the magnitude of the effect for the variable of sex and a decrease in prevalence ratio for the variable of age. Due to the theoretical importance, was decided to include in the final modeling the variable of skeletal muscle mass index, observing a statistically significant association with PA, as well as an increase in the magnitude of the effect when adjusted by gender and age. Thus, elderly patients with severe sarcopenia, according to the skeletal muscle mass index, showed 1.26 occurrence (PR: 1.26; 95% CI: 1.05 to 1.50) times higher PA below normal values when compared to older people who had adequate muscle reserves (Table 3).
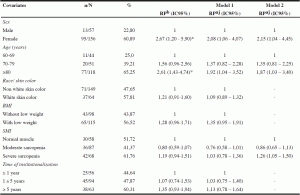
Table 3
Prevalence, ratio of gross and adjusted prevalence for an association between phase angle below normal values and covariates in institutionalized elderly of Salvador- BA, Brazil, 2012-2013
Note: n number of individuals with phase angle ranked below normal values; N – total number of individuals ; % – Prevalence ; PR – Prevalence ratio ; PRaj – adjusted prevalence ratio ; CI- confidence interval ; BMI- body mass index ; SMI- skeletal muscle index. Poisson regression model with adjusted Prevalence ratio and adjusted association between phase angle below the normal range and other variables. * P value ≤ 0.05
Discussion
This study evaluated the PA and the factors associated with their values in institutionalized elderly. It was found that men had higher PA values. This can be explained by skeletal muscle mass reserve to be higher in this group.
More than half of the elderly evaluated (50.7 %) had PA values below normal, however, it should be noted that the most frail elderly and probably with low PA were not included in the study, given the impossibility for the examination of bioelectrical impedance. Consequently, this percentage may be underestimated.
Among subjects with PA below normal values, female elderly, over 80 years stand out. Similar results were found by Dittamar (15), Barbosa -Silva et al. (1) and Bosy – Westphal et al. (3) in their population studies with healthy older Americans and Germans, respectively. The fact that women have higher longevity (16, 17), one of the hypotheses is that over the years, the elderly individual tends to have a higher burden of chronic diseases, as well as process influences aging in their health (18) reflecting, possibly in smaller PA values.
Another important finding relates to the reduction of PA with increasing age. It is known that, as the age increases there is a reduction of cellular and muscle mass, causing a decrease of reactance. It is also a decrease in the percentage of body water and fat tissue increased , increasing the resistance to the passage of electric current, in both sexes , justifying the results described above (1, 3, 15). Similar results were found in other studies with healthy elderly living in communities in Germany (3), Italy (19) and United States of America (1, 15) and institutionalized Italian elderly (20).
Another variable associated with PA below normal values was the severe sarcopenia, measured in this study by skeletal muscle mass index. We chose to use in the final model the variable skeletal muscle mass index due to the strong connection described in the literature between these variables, especially among the elderly, given that the PA values relates linearly with skeletal muscle mass independently and associated with several factors, including age (4, 20).
These results suggest that PA values may be interpreted as a skeletal muscle mass marker in the elderly, according to the findings in the literature that has proposed the PA as a predictor of muscle mass reserves and possibly nutritional and health status marker in this age population (2, 4, 19, 22).
In studies with non-institutionalized elderly was observed that the values of PA were determined, among other variables, by sex and age (1, 3, 15, 19). However, it is observed that the mean values of the PA of institutionalized elderly tend to be lower than those of non-institutionalized elderly (1, 3, 15,19), possibly due to the expected differences in terms of health and existing morbidities load between these two groups (23-24).
According Dittamar (15), PA values are higher in the presence of health preserved, proper nutrition and consistent physical exercise, conditions not always observed not always among the institutionalized elderly because they have a different health status in relation to the elderly living in the community.
This study is another contribution to knowledge about the PA behavior, recent field of study, particularly among the institutionalized Brazilian elderly population. The limitation of
this study refers to the likely underestimation of the prevalence of PA below normal values due to the inability of the elderly meet the criteria for performing the bioelectrical impedance and therefore not participate in this study. Thus, the high prevalence of PA below normal values observed between elderly may be arising both from a pathologic process, resulting from the above factors, but can also be a reflection of a physiological condition, mediated by the changes caused by the natural aging process, demonstrating the need for further studies in the area, for the enlightenment of those aspects related to low PA values.
Conclusion
The occurrence of PA below normal values was high and was associated with female sex, severe sarcopenia and longevity among the elderly, demonstrating the possible role of PA as a toll to identify elderly patients with compromised skeletal muscle mass reserve. However, it should be noted that the reduction of PA values in the evaluated elderly might reflect a physiological reduction of cell and muscle mass, inherent to the natural aging process, as well as the health commitment of these individuals, because of chronic diseases, common in this age group population.
Ethical standards: This study comply with the current laws of the country. The Research Ethics Committee of the Nutrition School of Federal University of Bahia, assent 11/12, approved the study. The results of evaluations were returned to institutions and when necessary the elderly were referred for treatment in outpatient nutrition and geriatrics of the Federal University of Bahia.
Funding: Funded by the Foundation for Research in the State of Bahia (FAPESB), Brazil (Grant Number 029/2013).
Conflict of interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
1. Barbosa-Silva, M. C. G., Barros, A. J., Wang, J., Heymsfield, S. B., & Pierson, R. N. Bioelectrical impedance analysis: population reference values for phase angle by age and sex. The American journal of clinical nutrition, 2005;82(1), 49-52.
2. Wilhelm-Leen, E. R., Hall, Y. N., Horwitz, R. I., & Chertow, G. M. Phase angle, frailty and mortality in older adults. Journal of general internal medicine, 2014;29(1), 147-154.
3. Bosy-Westphal, A., Danielzik, S., Dörhöfer, R. P., Later, W., Wiese, S., & Müller, M. J. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. Journal of Parenteral and Enteral Nutrition, 2006;30(4), 309-316.
4. Norman, K., Stobäus, N., Pirlich, M., & Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis–clinical relevance and applicability of impedance parameters. Clinical nutrition, 2012;31(6), 854-861.
5. Kyle, U. G., Bosaeus, I., De Lorenzo, A. D., Deurenberg, P., Elia, M., Gómez, J. M., … & Scharfetter, H. Bioelectrical impedance analysis—part I: review of principles and methods. Clinical nutrition, 2004;23(5), 1226-1243.
6. Baumgartner, R. N., Chumlea, W. C., & Roche, A. F. Bioelectric impedance phase angle and body composition. The American journal of clinical nutrition, 1998;48(1), 16-23.
7. Brazil. Brazilian Institute of Geography and Statistics, 2006. II Encontro Nacional de Produtores e Usuários de Informações Sociais, Econômicas e Territoriais. PhysicsWeb. http://www.ibge.gov.br/confest_e_confege/pesquisa_trabalhos/arquivosPDF/M705_01.pdf. Accessed 14 january 2015.
8. World Health Organization, 1995. Physical status: The use of and interpretation of anthropometry, Report of a WHO Expert Committee.
9. Jeliffe, D. B. The assessment of the nutritional status of the community with special reference to field surveys in developing regions of the world. WHO Monograph Series, 1966;(53).
10. Chumlea, W. C., Roche, A. F., & Steinbaugh, M. L. Estimating stature from knee height for persons 60 to 90 years of age. Journal of the American Geriatrics Society, 1985;33(2), 116-120.
11. Pan American Health Organization (PAHO), 2002. XXXVI Reunión del Comitê Asesor de Ivestigaciones en Salud – Encuestra Multicêntrica – Salud Beinestar y Envejecimeiento (SABE) en América Latina e el Caribe. Kingston, 2001. 93 p. PhysicsWeb. <http://envejecimiento.csic.es/documentos/documentos/paho-salud-01.pdf >. Acessed 19 march 2015.
12. Lauretani, F., Russo, C. R., Bandinelli, S., Bartali, B., Cavazzini, C., Di Iorio, A., … & Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. Journal of applied physiology, 2003;95(5), 1851-1860.
13. Janssen, I., Heymsfield, S. B., Baumgartner, R. N., & Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis.Journal of applied physiology, 2000;89(2), 465-471.
14. Janssen, I., Baumgartner, R. N., Ross, R., Rosenberg, I. H., & Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. American journal of epidemiology, 2004;159(4), 413-421.
15. Dittmar, M. Reliability and variability of bioimpedance measures in normal adults: effects of age, gender, and body mass. American journal of physical anthropology, 2003;122(4), 361-370.
16. Camarano, A. A., & Kanso, S. As instituições de longa permanência para idosos no Brasil. Revista brasileira de estudos de população, 2010;27(1), 232-235.
17. Camarano, A. A., & Mello, J. L. Cuidados de longa duração no Brasil: o arcabouço legal e as ações governamentais. Camarano AA. Cuidados de longa duração para a população idosa: um novo risco social a ser assumido, 2010;67-91.
18. Prince, M. J., Wu, F., Guo, Y., Robledo, L. M. G., O’Donnell, M., Sullivan, R., & Yusuf, S. The burden of disease in older people and implications for health policy and practice. The Lancet, 2015;385(9967), 549-562.
19. Buffa, R., Floris, G., & Marini, E. Assessment of nutritional status in free-living elderly individuals by bioelectrical impedance vector analysis.Nutrition, 2009;25(1), 3-5.
20. Buffa, R., Floris, G., & Marini, E. Migration of the bioelectrical impedance vector in healthy elderly subjects. Nutrition, 2003;19(11), 917-921.
21. Basile, C., Della-Morte, D., Cacciatore, F., Gargiulo, G., Galizia, G., Roselli, M., … & Abete, P. Phase angle as bioelectrical marker to identify elderly patients at risk of sarcopenia. Experimental gerontology, 2014;58, 43-46.
22. Norman, K., Smoliner, C., Valentini, L., Lochs, H., & Pirlich, M. Is bioelectrical impedance vector analysis of value in the elderly with malnutrition and impaired functionality?. Nutrition, 2007;23(7), 564-569.
23. Khoury, H. T. T., & NEVES, A. Percepção de controle e qualidade de vida: Comparação entre idosos institucionalizados e não institucionalizados.Rev. Bras. Geriatr. Gerontol. Rio de Janeiro, 2014;17(3), 553-565.
24. Silva, J. L., Marques, A. P. D. O., Leal, M. C. C., Alencar, D. L., & Melo, E. M. D. A. Factors associated with malnutrition in institutionalized elderly. Revista Brasileira de Geriatria e Gerontologia, 2015;18(2), 443-451.
